Abstract
Keratinocyte carcinomas (KCs) are now an epidemic in The United States of America, especially in elderly patients. KCs, including basal cell carcinoma and squamous cell carcinoma, can lead to disfigurement and occasionally death. However, the lower mortality rate associated with KC compared with melanoma allows for increased flexibility in the selection of treatment. Flexibility in treatment is particularly important in the elderly given that this patient population often has medical comorbidities that should be considered. These patients may have multiple KCs, higher risk tolerance to recurrence, and different concerns about cosmetic outcomes compared with their younger counterparts. We review treatment options for KCs and how the selection of each option may affect the elderly patient.
Keywords: Keratinocyte carcinoma, nonmelanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, elderly
Introduction
Keratinocyte carcinomas (KCs), commonly called nonmelanoma skin cancers (NMSCs), including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), are now an epidemic (Rogers et al., 2015, Samarasinghe and Madan, 2012). An estimated 5.4 million KCs were diagnosed in Americans in 2012 (Rogers et al., 2015). The incidence of BCC and SCC has increased by 145% between 1976 and 1984 and by 263% between 2000 and 2010, with women experiencing a greater increase in incidence rates for both BCC and SCC (Muzic et al., 2017). When considering the aging population and the fact that 80% of NMSC cases occur in people age ≥ 60 years, it is estimated that by 2030 the number of NMSC cases presenting to dermatologists could continue to increase by 50% (Diffey and Langtry, 2005).
Overall, KCs are associated with lower disease-specific mortality rates and a lower risk of metastatic spread compared with malignant melanoma (Thompson et al., 2016). However, high-risk cutaneous SCCs have a significant rate of nodal metastasis (3.7%-5.2%) and an absolute number of annual disease-specific deaths that is comparable with melanoma (Clayman et al., 2005, Karia et al., 2013, Samarasinghe and Madan, 2012, Thompson et al., 2016). High-risk KCs require more aggressive treatment, but the less lethal nature of low-risk KCs allows for greater flexibility in the management and selection of therapeutic modalities based on the type of KC, medical status of the patient, and the individual’s preferences. This article will review special considerations and treatment methods for KCs in elderly patients.
Consultation
Specific tumor characteristics must be considered when reviewing treatment options. High-risk tumors are associated with local recurrence, nodal metastasis, and death and therefore should be treated and monitored more aggressively (Puig and Berrocal, 2015, Thompson et al., 2016). For SCC and BCC, the generally agreed-upon characteristics of a high-risk tumor include tumor recurrence; diameter ≥ 2 cm; location on the vermillion lip, ear, mask areas of the face, hands, feet, or genitalia; thickness > 2 mm; poorly differentiated histology; or invasion of the subcutaneous tissue or structures, such as perineural, vascular, or lymphatic tissue (Baum et al., 2018, Bichakjian et al., 2017a, Bichakjian et al., 2017b, Parikh et al., 2014). Furthermore, KCs in anatomic locations, such as the eyelids, lips, or nose, or those that involve significant nerves or vessels may result in functional impairment, such as cranial nerve deficits, and should be treated accordingly (Mendenhall et al., 2012, Neville et al., 2007).
When analyzing treatment options for an elderly patient, the biological rather than chronologic age should be considered (Garcovich et al., 2017). The biological age (i.e., functional age) is largely determined by the patient’s physical and mental health, home environment, and family support, which are important factors to take into account when planning treatments to minimize negative outcomes (Garcovich et al., 2017).
Patients who may be at increased risk of adverse outcomes from treatment include those who may be considered “frail” with a myriad of symptoms, such as weakness, fatigue, weight loss, cognitive changes, and physical inactivity (Garcovich et al., 2017). Conditions to consider that may affect treatment decisions include those that may limit a patient’s life expectancy as well as comorbidities such as diabetes mellitus, venous or arterial disease, immunosuppression, malnutrition, presence of infection, and tobacco use, which can impede wound healing. The effects of comorbidities on treatment decisions are specifically discussed in the context of treatment options in the following sections.
Each treatment option and relevant considerations for elderly patients is outlined in the following sections, including active surveillance; surgical management, including Mohs micrographic surgery (MMS) and wide local excision (WLE); superficial ablative procedures, including electrodessication and curettage (ED&C), cryotherapy, radiation therapy (RT), and photodynamic therapy (PDT); topical treatments; and targeted drug therapies. The treatment options discussed are commonly used but not all-inclusive and may be varied or combined on the basis of patient and provider preferences.
Active surveillance
KCs typically have an indolent disease course and may go unnoticed by the patient. For patients with a limited life expectancy or significant comorbidities, a valid option may be to forgo treatment; KC is typically nonfatal when discovered at the end of life (Jung and Linos, 2016, Linos, 2013). A low recurrence risk of 7.2% at 40 months was demonstrated by Reiger et al. (2010) in patients with low-risk BCCs and SCC in situ (SCCis) that were minimally transected at the time of diagnosis. These data suggest that active surveillance may be an option for some elderly patients who are unable or unwilling to undergo any type of treatment. Clinical and histologic evaluation must lead this discussion. Caution should be taken with lesions that show substantial malignancy, show histologically positive deep or lateral margins, or are located on the head and neck (specifically the nose, ears, lips, and eyelids). Such tumors can become progressively more symptomatic and cosmetically disfiguring and can require more extensive procedures or treatment if they advance. Additionally, if the patient is immunosuppressed, active surveillance is usually not recommended because these patients often have more aggressive KCs than the general population, leading to greater morbidity and mortality (Brin et al., 2014).
Given the current medical environment, detailed documentation with regard to the discussion about treatment options and alternatives and the patient’s election to forgo treatment should be entered into the health record. However, this option should involve routine follow-up office visits with the dermatologist that may be burdensome to patients if they require assistance with transportation. If circumstances change or the lesion becomes symptomatic, the patient always has the option to elect for treatment at a later time.
Surgical management (Mohs micrographic surgery and wide local excision)
Surgical management is often the standard therapy for the removal and cure of KCs. These treatment options (WLE and MMS) are invasive, but they provide the highest cure rates. The 5-year cure rates for MMS are approximately 99% and 97% for primary BCC and SCC, respectively (Giordano Resti et al., 2014, Kauvar et al., 2015a, Kauvar et al., 2015b, Leibovitch et al., 2005). This number drops to around 90% to 93% for bulkier, more aggressive, and recurrent BCC, 90% for previously treated, recurrent SCC and 67.4% for poorly differentiated SCC (Bhatnagar et al., 2016, Rowe et al., 1992, Smeets et al., 2004, Wennberg et al., 1999).
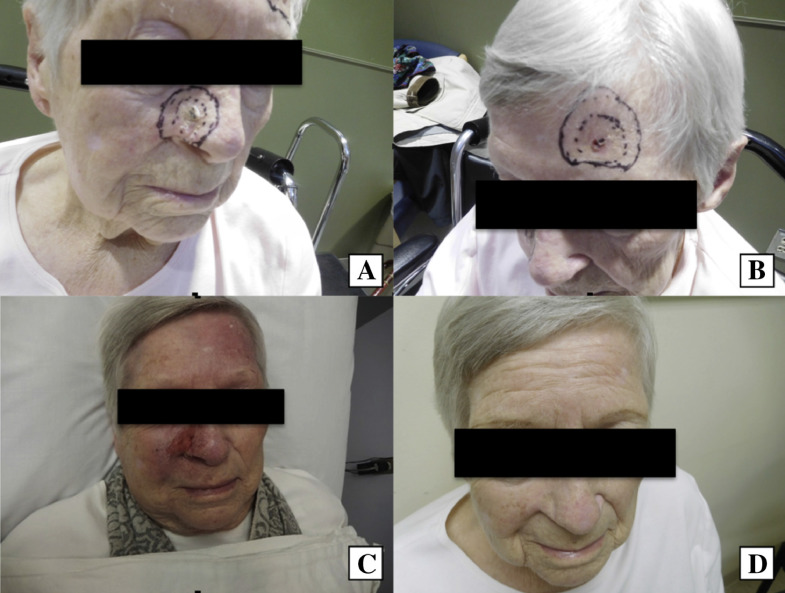
The cure rates with WLE of SCCs and low-risk BCCs are approximately 91% and 95% to 98%, respectively (Bichakjian et al., 2017a, Bichakjian et al., 2017b). MMS should always be considered as a means of treatment in anatomic sites with a high risk of recurrence, lesions with poorly defined margins, tumors located on previously irradiated skin, or lesions located on areas in which conservation of tissue is vital (Fig. 1; Prickett and Ramsey, 2017, Shriner et al., 1998). Alternatively, WLE may be used for lesions where tissue sparing is less critical.
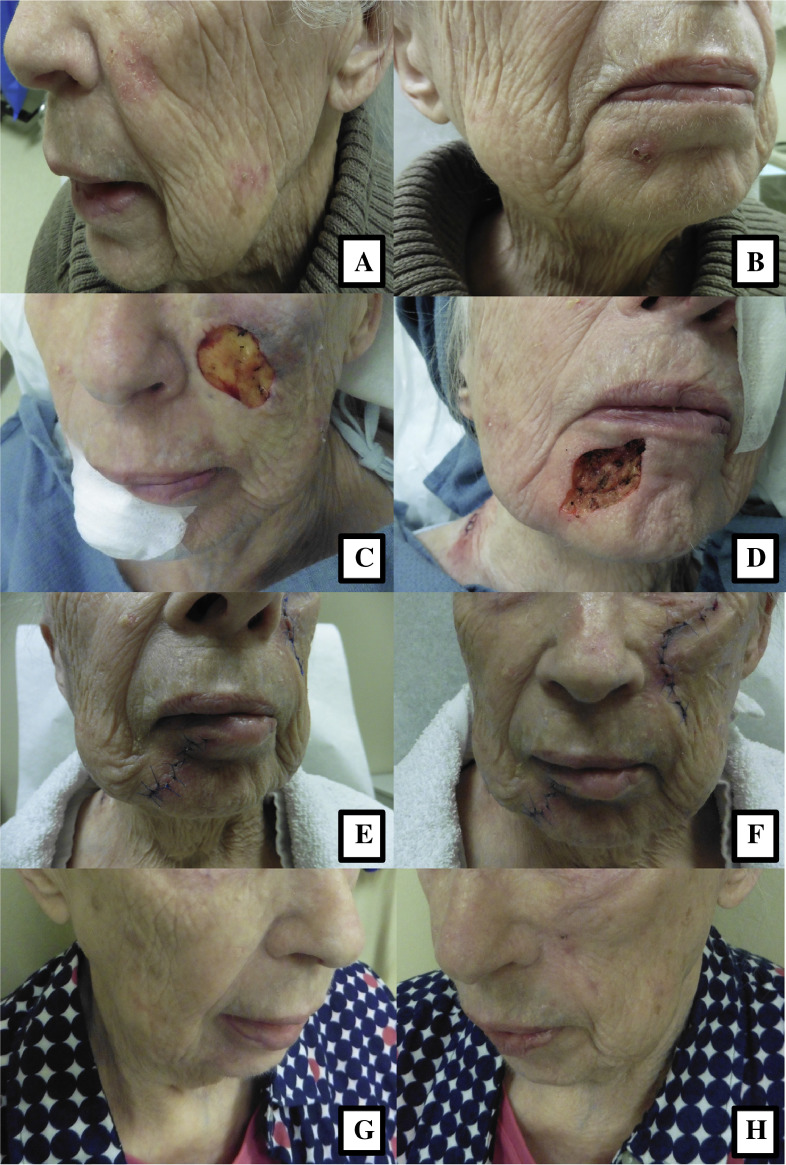
Fig. 1.
An 86-year-old woman with (A) poorly defined squamous cell carcinoma of the left medial cheek and (B) basal cell carcinoma of the right chin treated with Mohs micrographic surgery (C, D) and primary reconstruction (E, F). Two months postoperatively, the patient had an excellent cosmetic outcome (G, H).
There is a misconception that MMS is a lengthy procedure that may be unsafe for elderly patients, and the word “surgery” may dissuade patients from pursuing this gold-standard treatment option. Studies have shown no correlation between age and adverse outcomes or patient satisfaction, and most patients return home within a few hours (Hussain et al., 2017). Generally, WLE and MMS provide excellent cosmetic outcomes (Cumberland et al., 2009, Divine et al., 2015). Additionally, these may be the best options for patients who desire reassurance of cancer eradication because they both involve margin assessment.
The risks of MMS and WLE include infection, bleeding, bruising, wound dehiscence, permanent cosmetic change, and nerve or sensation issues. However, the overall complication rate for skin surgery is < 2% (Cook and Perone, 2003). Surgical procedures are inherently associated with a variable degree of acute postoperative pain, which is often sufficiently controlled with supportive care in most patients (Glass et al., 2015). Additional short-term disadvantages include physical activity restrictions, postoperative discomfort, wound care responsibilities, and the potential need for additional follow-up visits for suture removal or wound evaluation. This may not be feasible for patients who live at a greater distance from the treatment facility.
Wound care and follow-up visits for suture removal can be minimized by using absorbable sutures in both the dermal and epidermal layers for closure of the surgical defect and coverage with wound-closure tape or transparent dressing to protect the incision (Whitely et al., 2003). Additionally, in appropriately selected cases, surgical defects after MMS may be allowed to heal via secondary intention to decrease operative time and simplify wound care (Whitely et al., 2003). The utilization of allografts with or without purse-string suturing after MMS or the excision of large surgical defects in the elderly can also be a reconstructive option that can minimize wound care burden. (Lyons et al., 2018, Park et al., 2016)
When considering appropriate candidates for surgical treatment, patient comorbidities, cognitive function, lesion location, and daily medications should be taken into account. Immunosuppression or an impaired ability to care for postoperative wounds increases the risk for postoperative infection, poor wound healing, and scarring (Guo and DiPietro, 2010). Lesions, especially on the lower extremities, may cause delayed or adversely affected healing after surgery due to underlying diabetes mellitus, poor circulation, smoking, malnutrition, and poor baseline hygiene (Guo and DiPietro, 2010). Antiplatelet and anticoagulant drugs, herbal or nutritional supplements with similar blood thinning effects, and the ingestion of alcohol increase the risk of intraoperative bleeding and may adversely affect postprocedural hemostasis, wound healing, and scar formation (Guo and DiPietro, 2010, Stanger et al., 2012). Patients on these medications should be advised about their potential effects on surgical outcomes. Nonmedically prescribed or nonessential products should be discontinued at least 1 week before surgery (Bunick and Aasi, 2011), and medically prescribed blood thinners and anticoagulants ideally should be continued when possible, especially in patients with a history of myocardial infarction, stroke, blood clots, and cardiac arrhythmias (Alcalay and Alkalay, 2004, Callahan et al., 2012). Measures such as the direct placement of an absorbable sterile gelatin sponge along the incision and extended application of pressure bandaging can help mitigate postoperative bleeding in anticoagulated patients.
Electrodessication and curettage
ED&C is a minor surgical procedure used for the treatment of low-risk BCCs and SCCs. The cure rates of ED&C are the highest for low-risk BCC but can be as high as 95% for both low-risk BCC and SCC with appropriate tumor selection (Fig. 2; Bichakjian et al., 2017a, Bichakjian et al., 2017b, Chren et al., 2013, Marzuka and Book, 2015, Sheridan and Dawber, 2000).
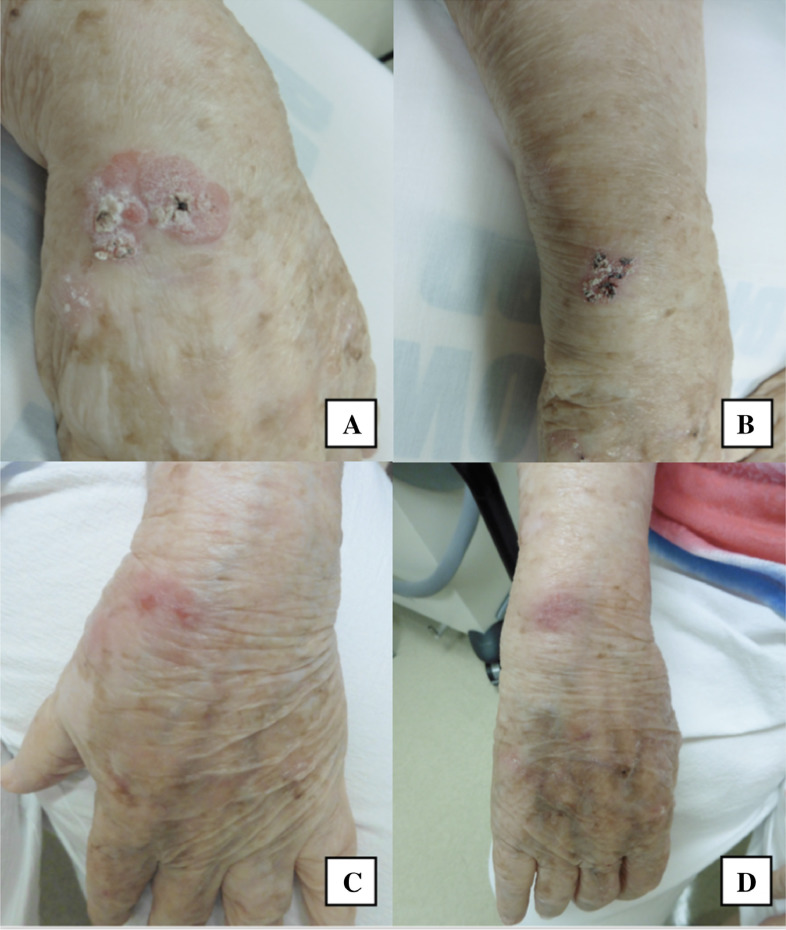
Fig. 2.
An 89-year-old woman with squamous cell carcinoma in situ of the left (A) and right (B) wrist treated with electrodessication and curettage and at follow-up 1 month after treatment (C, D).
The advantages of ED&C include a single, minimally invasive, brief visit for treatment, with minimal intraoperative and postoperative pain, down time, and postprocedural follow-up visits. The risk of infection and bleeding are also lower compared with wounds closed with suture (Divine et al., 2015). ED&C wounds typically heal in 4 to 6 weeks (Bolotin and Alam, 2015).
Disadvantages include flat, round, scars that may become hypopigmented, atrophic, or hypertrophic and are typically considered less cosmetically acceptable than linear surgical excision scars. Therefore, ED&C is generally avoided in highly cosmetically sensitive areas such as the face (Galles et al., 2014). A study on quality of life in patients with an average age of 65 years who were treated with WLE, MMS, or ED&C revealed improved quality of life after WLE and MMS but not ED&C (Chren et al., 2007). Although the relatively brief duration of the ED&C procedure and the potentially decreased risk of postprocedural bleeding may be appealing to some patients, healing time is not always reduced compared with primary linear closures, especially when Mohs tissue-sparing procedures are performed. ED&C is also not an ideal treatment in terminal hair-bearing areas because the tumor may extend down the follicles (Bichakjian et al., 2017a, Bichakjian et al., 2017b). ED&C does not allow for histologic margin assessment to confirm tumor eradication after treatment and is operator-dependent with higher cure rates produced by more experienced clinicians (Bichakjian et al., 2017a).
In an elderly population, it is important to consider the presence of implanted electrical devices, such as pacemakers, defibrillators, deep-brain stimulators, cochlear implants, nerve stimulators, and gastric pacemakers given the potential for high-frequency electrical current from the cautery source to cause device malfunction (Bolotin and Alam, 2015). Many variables contribute to whether electrodessication can be safely used, such as site distance from the device, length of time of electrocautery usage, and the qualities of the implanted device itself (Bolotin and Alam, 2015).
Curettage alone has comparable cure rates with ED&C for BCC and well-differentiated SCC despite the theorized increased cure rates from heat and the incited inflammatory destruction of tumor cells due to electrodessication (Barlow et al., 2006, Yakish et al., 2017). Thus, alternative machines, battery-powered heat electrocautery or bipolar forceps, or chemical cautery with agents including aluminum chloride, Monsel’s solution, and silver nitrate can be used for hemostasis without implanted device interference or a reduction in cure rate.
Superficial ablative procedures
Procedures including cryotherapy, RT/brachytherapy (BT), and PDT may be good options for patients who are poor candidates for surgery, have many complicating comorbidities, or may not desire surgical treatment. The specific advantages and disadvantages of each treatment modality are outlined in the following, but the disadvantages inherent to all nonsurgical treatments are a lower cure rate and lack of histologic confirmation of tumor eradication after treatment.
Cryotherapy
Cryotherapy is a superficially ablative procedure that uses liquid nitrogen to damage the cancerous tissue with intracellular ice formation, with slow thaw times and repeat freeze-thaw cycles producing increasing tissue injury that is necessary for the treatment of malignancies (Andrews, 2004).
The recurrence rates for BCCs treated with cryotherapy are variable and range from 0% to 39% (Bichakjian et al., 2017a). Prospective and retrospective studies have shown recurrence rates of 0% to 4% for invasive SCCs treated with cryotherapy. For SCCis, the recurrence rates after cryotherapy range from 1% to 13% in retrospective studies and 0% to 50% in prospective studies (Bichakjian et al., 2017b).
The wide range of reported recurrence rates likely reflects the variability in tumor selection and differences in treatment technique. Various individual characteristics related to the patient, tumor, and provider can affect the efficacy of cryotherapy and should be considered before choosing cryotherapy as a treatment option. Although thermocoupling needles may be used to measure the base temperature of the tumor, with a goal of –50°C, this is rarely done in the clinical setting today. Practitioners instead typically rely on freeze times of 60 to 90 seconds for BCCs up to 1.5 cm in diameter (longer for larger lesions) and a 3 to 5 mm freeze zone margin around the lesion. Lesions should be allowed to thaw spontaneously with thaw times of approximately three times the freeze time (Savant et al., 2017).
The advantages of cryotherapy include its quick, inexpensive, and noninvasive nature, as well as its low rates of postprocedure infection and bleeding (Andrews, 2004, Bichakjian et al., 2017b). Local anesthesia is not needed, minimal posttreatment care is required, and no specific procedure-related follow-up vists are necessary (Bichakjian et al., 2017b). Patients are often familiar with cryotherapy for precancerous lesions and are frequently interested in cryotherapy when a noninvasive procedure is desired (Fig. 3).
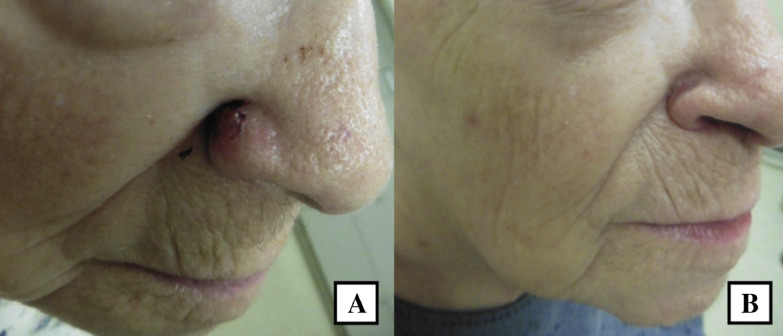
Fig. 3.
A 92-year-old woman with a nodular and infiltrative basal cell carcinoma of the right ala (A) treated with double freeze-thaw cryotherapy and at follow-up 7 weeks later (B).
The disadvantages of cryotherapy include its common, acute side effects, including erythema, blister formation, edema, pain, and headaches if applied to facial lesions (Fig. 4; Andrews, 2004). A burning sensation of variable intensity is often described by patients and is most intense on the ears, temples, and fingertips (Andrews, 2004). Cryotherapy has been found to be more painful than ED&C and takes longer to heal than ED&C and sutured wounds (Bichakjian et al., 2017b, Kauvar et al., 2015a). Cryotherapy is also a very operator dependent procedure, and the results may be inadequate if the tumor is undertreated. Other treatment options may be considered if the patient has very low pain tolerance. Scarring from cryotherapy may be more pronounced compared with topical chemotherapeutic regimens; if aggressively performed, cosmesis can be inferior to other treatment options (Bichakjian et al., 2017a). Cryotherapy can also lead to local cicatrizing alopecia when used on hair-bearing sites (Zouboulis, 2015).
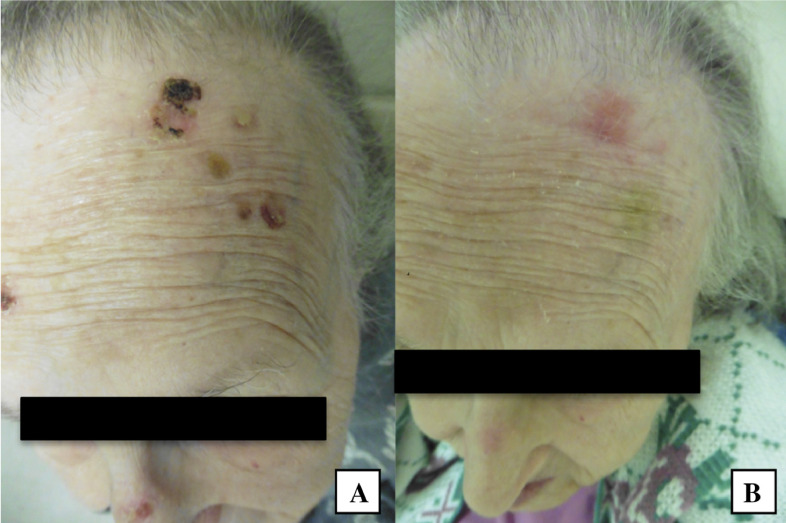
Fig. 4.
A 91-year-old woman with a squamous cell carcinoma in situ of the frontal scalp (A) treated with double freeze-thaw cryotherapy and at follow-up 4 weeks later (B).
Relative contraindications include cold urticaria, Raynaud’s disease, cryoglobulinemia, collagen disease, multiple myeloma, and pyoderma gangrenosum (Andrews, 2004).
Radiation therapy
RT is a noninvasive treatment option for the management for KCs. Options include external beam RT and high-dose-rate (HDR) BT. HDR-BT offers the benefit of convenience over other forms of RT and a compressed treatment schedule of 5 to 10 sessions as opposed to external beam RT, which may require 20 to 30 daily treatments (Bhatnagar et al., 2016, Kohler-Brock et al., 1999). Electronic BT and superficial RT devices use a low-energy x-ray source rather than an ionized source and allow for minimal protective shielding requirements and tighter treatment margins (Ballester-Sánchez et al., 2017, Bhatnagar et al., 2016).
When used to treat primary BCCs, RT allows for a 5-year local control rate of 93% to 96% (Bichakjian et al., 2017a). Tumors that are smaller in size and of the nodular subtype have enhanced responsiveness to RT (Bichakjian et al., 2017a). Recurrent lesions are more difficult to treat, resulting in an 80% control rate (Locke et al., 2001). Treatment of primary SCCs with RT produces cure rates of 90% to 93% (Bichakjian et al., 2017b). Local control rates of KCs treated with ionized (iridium) or electronic HDR-BT are high, ranging from 72% to 100%, and many studies report success rates of well over 90% (Ballester-Sánchez et al., 2017, Delishaj et al., 2016, Olek Jr et al., 2018, Tormo et al., 2014).
Superficial RT machines, which are electronic BT systems such as the SRT-100 (Sensus Healthcare, Boca Raton, FL) or Axxent eBx System (Xoft, Inc, San Jose, CA), have become increasingly available in dermatology offices for the treatment of smaller, thinner, less aggressive NMSCs (Cognetta Jr et al., 2016, David, 2013). Data on short-term recurrence rates and cosmetic outcomes are promising, but data on long-term efficacy are very limited (David, 2013, Grant-Kels and VanBeek, 2014).
The advantages of RT include noninvasive, quick, and painless treatment sessions and minimal aftercare. RT may be particularly useful for patients who are frail, on multiple anticoagulants, have poor wound healing capacity, or cannot properly perform wound care (Bhatnagar et al., 2016). Patients with dementia, anxiety, or other neurologic or psychiatric disorders who are unable to undergo surgical procedures may be good candidates for RT (Bhatnagar et al., 2016). There is also a special role for RT in simple palliation of symptomatic lesions in patients with a limited life expectancy (Vuong et al., 2017).
Disadvantages of RT include cutaneous, radiation-induced side effects and very frequent (daily to bi-weekly) appointments for several weeks, which can be very burdensome for elderly patients, especially those who live far away from treatment centers and do not drive. (Ballester-Sánchez et al., 2017). Common acute adverse effects include radiation dermatitis, pruritus, ulcerations, desquamation, and erythema. Delayed side effects can appear months after treatment and may include dyspigmentation, alopecia with scalp lesions, atrophy, and telangiectasias, which may affect cosmetic favorability (Ballester-Sánchez et al., 2017, Delishaj et al., 2016, Hulyalkar et al., 2011, Kauvar et al., 2015a). Due to concern for long-term complications, including radiation-induced fibrosis, chronic dermatitis, and secondary cutaneous malignancies, RT is generally reserved for elderly patients and avoided in younger patients with a longer life expectancy (Bichakjian et al., 2017a, Bray et al., 2016). In addition, tumors that recur after RT tend to be highly infiltrative and aggressive (Kauvar et al., 2015a, Kauvar et al., 2015b).
Furthermore, eradication of the complete tumor is not histologically confirmed with RT (Kauvar et al., 2015a). NMSCs are frequently composed of > 1 histologic subtype. One study found that 21.1% of biopsies under diagnosed the aggressiveness of BCCs and SCCs when compared with subsequent MMS pathology samples, owing to the heterogeneity of many tumors and lack of complete tumor visualization with biopsy (Izikson et al., 2010). Therefore, if the overall pathology or base of the tumor differs from the pathology of the biopsy sample, this may affect risk stratification and reduce treatment success (Bartoš and Kullová, 2016, Cortés-Peralta et al., 2018, Sexton et al., 1990).
RT can be a beneficial option for nonsurgical candidates who have skin cancers located in areas near or involving tendons or bones or on areas with a complex topography, such as the face and hands (Fig. 5; Aldelaijan et al., 2017, Patel et al., 2017). RT can also be used as an adjunct to surgery for very bulky or high risk of recurrence tumors as well as those with positive margins, bony or perineural invasion, or in instances of recurrence (Hulyalkar et al., 2011). Ultimately, RT would be a great option for large or rapidly growing tumors that are potentially disfiguring or may otherwise involve extensive surgeries. One should consider the patient holistically (advanced age, comorbidities, and preferences) when deciding whether RT is a desirable treatment option; some clinicians prefer to reserve RT for patients in their late 70s or > 80 years old.
Fig. 5.
A 90-year-old woman with a basal cell carcinoma of the right ala (A) and left forehead (B) treated with high-dose-rate brachytherapy at completion of therapy (C) and at 5-month follow-up (D).
Photodynamic therapy
PDT is an effective, noninvasive option for the treatment of superficial BCCs (sBCCs) and nodular BCCs (nBCCs) as well as SCCis (Cohen and Lee, 2016). PDT is most effective in treating lesions < 2 mm in thickness and < 2 cm in size (Ahmadi et al., 2004, Ozog et al., 2016). The treatment of thicker lesions should include debulking prior to PDT with possible methods including gentle curettage or tape stripping prior to photosensitizer application or occlusion of topical keratolytics the night before (Bay et al., 2017, Gerritsen et al., 2009, Ozog et al., 2016). Treatment of sBCCs and nBCCs leads to clearance rates ranging from 70% to 90% (Bichakjian et al., 2017a, Savoia et al., 2015) after one or two PDT treatments with gentle curettage for nBCCs. Higher cure rates are seen in sBCCs versus nBCCs (Bichakjian et al., 2017a). SCCis treated with PDT results in an initial clearance between 52% and 98% (Bichakjian et al., 2017b) and may reflect differences in treatment technique (Bichakjian et al., 2017b).
PDT is a noninvasive technique that requires a limited number of treatments, effectively covers a large surface area, and does not rely on patient compliance with at-home treatments (Braathen et al., 2007, Rkein and Ozog, 2014). In general, PDT is very well tolerated with a short healing time, minimal wound care requirements, and outstanding cosmetic outcomes (Bichakjian et al., 2017a, Steinbauer et al., 2010).
PDT is less efficacious when treating thick or ulcerated KCs due to limited penetration of the photosensitizing agent, limiting its treatment to KCs previously listed (Matei et al., 2013, Souza et al., 2007, Smucler and Vlk, 2008). Treatment requires patients to wait in the office for ≥ 1 hours on treatment days to allow for incubation of the photosensitizer (Braathen et al., 2007, Rkein and Ozog, 2014). A major disadvantage is pain during the illumination phase that can be severe and may lead to truncated treatment sessions and patient refusal for further treatments (Cohen and Lee, 2016). Interventions that are effective in reducing associated pain include the addition of cooling devices, pauses during treatment, inhaled nitrous oxide/oxygen during treatment, and pulsed dye laser coherent light compared with light-emitting diode noncoherent light (Cohen and Lee, 2016). The use of topical anesthetics has not been shown to be effective (Cohen and Lee, 2016, Fink et al., 2015). Additionally, patients with mental or physical limitations may have difficulties with the tight physical constraints of the light unit.
Common, less severe, side effects include burning, prickling, and stinging sensations during treatment, followed by the development of temporary erythema and edema immediately after treatment (Cohen and Lee, 2016, Lehmann, 2007). PDT is less likely than topical therapy to cause moderate-to-severe local swelling, erosion, crust formation, itching, and wound infections (Bichakjian et al., 2017a). Dyspigmentation after treatment is possible but occurs in < 5% of patients (Cohen and Lee, 2016). After treatment, patients are required to avoid sunlight for 2 days and should continue strict photoprotection until the treatment area is healed (Rkein and Ozog, 2014). Repeat treatments for full resolution of the lesion may be needed weeks to months after the initial treatment (Rkein and Ozog, 2014).
Topical agents
Topical anti-neoplastic agents are options for patients who may be poor candidates for surgery and those who are elderly, unhealthy, or have KCs in cosmetically sensitive areas (Micali et al., 2014). Topical agents are limited in their ability to penetrate the skin, and limited evidence exists for the use of topical agents to treat KCs beyond small, superficial tumors including sBCC and SCCis (Micali et al., 2014), so tumor type and size must be considered when contemplating the use of topical agents.
Commonly used and approved topical agents include 5-fluorouracil (5-FU) and imiquimod (IQ) (Micali et al., 2014). Proper application of 5-FU results in a brisk inflammatory reaction with mild oozing and crusting in the affected area, which is desired and represents a clinical endpoint of treatment (Love et al., 2009). 5-FU 5% cream applied twice daily for 6 weeks to treat sBCCs provided an 80% clearance rate (Arits et al., 2013). Limited evidence exists for the effective use of 5-FU in other subtypes of BCCs (Micali et al., 2014) with variable clearance rates (27%-93%) when used to treat SCCis (Bichakjian et al., 2017b).
IQ was approved by the U.S. Food and Drug Administration (FDA) for the treatment of sBCC in immunocompetent adults with tumors > 0.5 cm2 in area and < 2 cm in diameter and located on the trunk and extremities (Lien and Sondak, 2011). IQ is often favored for the treatment of BCCs over 5-FU because it has had higher and more durable clearance rates for sBCCs. IQ is reported to have an 85% 5-year disease-free rate when used once daily for 6 weeks (Bichakjian et al., 2017a, Quirk et al., 2010) and an 84% clinical clearance at 3 years when used once daily for 12 weeks to treat small nBCCs (Bath-Hextall et al., 2014, Bichakjian et al., 2017a).
Alternative dosing for IQ is a once-daily application to the affected site 5 days of the week (often Monday through Friday, with a break on the weekend) for 12 weeks, which provides an 80% clearance rate for sBCCs < 2 cm and 70% clearance rate for small (< 1.5 cm) nBCCs (Micali et al., 2014). Higher clearance rates may be obtained by twice daily dosing every day for 12 weeks, but the associated inflammation and discomfort is often too significant for patients to tolerate, especially in the elderly population (Bubna, 2015). IQ can be considered for treatment of SCCis, providing clearance rates of 70% to 100% depending on dosing schedules and with low recurrence rates (Bichakjian et al., 2017b, Chitwood et al., 2013, Love et al., 2009, Mackenzie-Wood et al., 2001, Peris et al., 2006, Rosen et al., 2007, Warshauer and Warshauer, 2008).
The advantages of treatment with topical agents include convenience (at-home application), tissue-sparing and noninvasive treatment, improved cosmetic outcome, minimal wound care and downtime, fewer follow-up office visits during treatment, and ease of use for patients with multiple comorbidities. The local side effects from IQ and 5-FU are similar; both should produce a local skin reaction with irritation, which is desired and expected. More intense reactions are associated with higher clearance rates (Fox et al., 2018, Love et al., 2009, Soyer et al., 2018). Both topical agents lead to erythema, which typically subsides in approximately 2 weeks but may last 12 weeks after treatment is discontinued (Love et al., 2009).
Almost all patients will experience at least one adverse effect during treatment, which may include erythema, pain, blistering, local swelling, irritant dermatitis, and pruritus ranging from mild to severe and can persist as long as 6 weeks after treatment (Love et al., 2009). These adverse events will cause a small percentage of patients (3% for IQ and 5% for 5-FU) to discontinue treatment prematurely, which may result in lower clearance rates (Bichakjian et al., 2017b, Love et al., 2009). Although low (0.01%), the risk of infection is higher with 5-FU and IQ than with PDT (Arits et al., 2013). Systemic side effects are uncommon, but 0.4% to 2% of patients report flu-like symptoms with the use of IQ (Arits et al., 2013, Fox et al., 2018). Dyspigmentation after treatment has also been reported (Fox et al., 2018).
Commonly used dosing schedules of IQ for sBCCs include once-daily applications for a minimum of 6 weeks or the FDA-recommended dosing of once-daily applications for 5 days a week for 6 to 16 weeks to balance treatment efficacy and side effect severity (Geisse et al., 2004, Love et al., 2009, Peris et al., 2006, Raasch, 2009, Rosen et al., 2007). The FDA-approved regimen for topical 5-FU in the treatment of sBCC is twice-daily application for 3 to 6 weeks irrespective of the tumor size or location; however, in the peer-reviewed literature, the average treatment length was significantly longer than that recommended by the FDA (11 vs. 3-6 weeks; Love et al., 2009).
Although not FDA approved, 5-FU may be used off label to treat SCCis. Dosing regimens vary on the basis of clinician preferences. An example of a reasonable dosing schedule may be application of 5-FU twice daily for 4 weeks with re-evaluation 4 weeks after completion of treatment. Patients who may not be able to be compliant with the treatment regimen or dosing schedule due to mental or physical limitations may not be good candidates for topical therapy (Bahner and Bordeaux, 2013). Barriers to compliance in the elderly include physical dexterity for medication application, ability to access the treatment area or regular help from a caretaker, and the capacity to understand and comply with the recommended application instructions and dosing schedules. Patients must also understand that electing to treat with a topical agent will require close long-term clinical follow-up to monitor for clearance and/or recurrence (Micali et al., 2014).
Patients with preexisting autoimmune diseases, such as psoriasis, myasthenia gravis, and HLA B27 spondyloarthropathy, may not be ideal candidates for treatment with IQ in light of reports of disease exacerbations during treatment with IQ, which are thought to be secondary to its immune-stimulating effects (Benson, 2004, Saad et al., 2017, Wu et al., 2004).
Targeted drug therapies
Patients with many smaller concurrent BCCs, BCCs located on anatomically challenging areas (e.g., large advanced tumors on the eyelids, nose, or ears), a very large tumor burden, or advanced disease may benefit from a hedgehog inhibitor, which may be safer and more tolerable than other treatment options (Basset-Séguin et al., 2017). Hedgehog inhibitors, such as vismodegib, are used to treat advanced BCCs through the deactivation of the hedgehog pathway, which can lead to shrinkage or possible clearance of the tumors (Ally et al., 2014). Side effects include alopecia, muscle spasms, weight loss, taste disturbances, fatigue, anorexia, nausea, diarrhea, and arthralgias, which can lead to discontinuation of the medication (Becker et al., 2017, Fellner, 2012). Drug holidays, intermittent dosing, or decreased dosages (i.e., lower than FDA-approved dosages) can increase drug tolerability, and abate or reverse most side effects without sacrificing efficacy (Becker et al., 2017, Dréno et al., 2017, Yang and Dinehart, 2016). More clinical data are needed to determine the best intermittent drug regimen.
For aggressive, metastatic, inoperable, or incompletely resected SCCs, preliminary data for programmed-death receptor 1 checkpoint inhibitors (e.g., pembrolizumab or nivolumab) show early promise with an excellent side effect profile, whereas traditional chemotherapies (e.g., platinum agents) show subpar response rates (Blum et al., 2018, Jarkowski 3rd et al., 2016, Tran et al., 2017).
Conclusion
As the geriatric population continues to live longer, the incidence of KCs in the elderly will also increase. Understanding the clinical indications and utility of available treatment options for KCs and their expected course and outcomes is essential for physicians who manage KCs. This is especially crucial when caring for elderly patients who have a wide variety of treatment needs and goals that require in-depth discussions of options beyond the standard of surgery and individualized, comprehensive treatment plans.
In recent years, patient-reported quality of life has emerged as an increasingly important assessment of the effects of various illnesses and their corresponding treatments. From preliminary studies, KC does not appear to greatly affect patients’ quality of life (Garcovich et al., 2017). However, few studies have evaluated the impact of various treatment options for NMSC on the quality of life in the elderly. Factors associated with each treatment option may affect quality of life, and thus treatment decisions, including postsurgical cosmesis, duration of procedure, and postprocedure downtime. Likely, each of these factors holds varying levels of importance based on patients’ age. Future work in this area is warranted to be able to consider intra- and posttreatment quality-of-life measures in treatment choice.
Acknowledgements
The authors thank Drs. Bahman Emami, Courtney Hentz, and Michael Mysz for their guidance and clinical photographs with regard to treatment with RT.
Footnotes
Sources of support: The open access fee for this article was sponsored by La Roche-Posay.
Conflicts of interest: None.
References
- Ahmadi S., McCarron P.A., Donnelly R.F., Woolfson A.D., McKenna K. Evaluation of the penetration of 5-aminolevulinic acid through basal cell carcinoma: A pilot study. Exp Dermatol. 2004;13(7):445–451. doi: 10.1111/j.0906-6705.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- Alcalay J., Alkalay R. Controversies in perioperative management of blood thinners in dermatologic surgery: Continue or discontinue? Dermatol Surg. 2004;30(8):1091–1094. doi: 10.1111/j.1524-4725.2004.30333.x. [DOI] [PubMed] [Google Scholar]
- Aldelaijan S., Bekerat H., Buzurovic I., Devlin P., DeBlois F., Seuntjens J. Dose comparison between TG-43-based calculations and radiochromic film measurements of the Freiburg flap applicator used for high-dose-rate brachytherapy treatments of skin lesions. Brachytherapy. 2017;16(5):1065–1072. doi: 10.1016/j.brachy.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Ally M.S., Tang J.Y., Joseph T., Thompson B., Lindgren J., Raphael M.A. The use of vismodegib to shrink keratocystic odontogenic tumors in patients with basal cell nevus syndrome. JAMA Dermatol. 2014;150(5):542–545. doi: 10.1001/jamadermatol.2013.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews M. Cryosurgery for common skin conditions. Am Fam Physician. 2004;69(10):2365–2372. [PubMed] [Google Scholar]
- Arits A.H., Mosterd K., Essers B.A., Spoorenberg E., Sommer A., De Rooij M.J. Photodynamic therapy versus topical imiquimod versus topical fluorouracil for treatment of superficial basal-cell carcinoma: A single blind, non-inferiority, randomized controlled trial. Lancet Oncol. 2013;14:647–654. doi: 10.1016/S1470-2045(13)70143-8. [DOI] [PubMed] [Google Scholar]
- Bahner J.D., Bordeaux J.S. Non-melanoma skin cancers: Photodynamic therapy, cryotherapy, 5-fluorouracil, imiquimod, diclofenac, or what? Facts and controversies. Clin Dermatol. 2013;31(6):792–798. doi: 10.1016/j.clindermatol.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Ballester-Sánchez R., Pons-Llanas O., Candela-Juan C., de Unamuno-Bustos B., Celada-Alvarez F.J., Tormo-Mico A. Two years results of electronic brachytherapy for basal cell carcinoma. J Contemp Brachytherapy. 2017;9(3):251–255. doi: 10.5114/jcb.2017.68191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow J.O., Zalla M.J., Kyle A., DiCaudo D.J., Lim K.K., Yiannias J.A. Treatment of basal cell carcinoma with curettage alone. J Am Acad Dermatol. 2006;54(6):1039–1045. doi: 10.1016/j.jaad.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Bartoš V., Kullová M. Basal cell carcinoma of the skin with mixed histomorphology: A comparative study. Cesk Patol. 2016;52(4):222–226. [PubMed] [Google Scholar]
- Basset-Séguin N., Hauschild A., Kunstfeld R., Grob J., Dréno B., Mortier L. Vismodegib in patients with advanced basal cell carcinoma: Primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334–348. doi: 10.1016/j.ejca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- Bath-Hextall F., Ozolins M., Armstrong S.J., Colver G.B., Perkins W., Miller P.S. Surgical excision versus imiquimod 5% cream for nodular and superficial basal-cell carcinoma (SINS): A multicentre, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15(1):96–105. doi: 10.1016/S1470-2045(13)70530-8. [DOI] [PubMed] [Google Scholar]
- Baum C.L., Wright A.C., Martinex J.C., Arpey C.J., Brewer J.D., Roenigk R.K. A new evidence-based risk stratification system for cutaneous squamous cell carcinoma into low, intermediate, and high risk groups with implications for management. J Am Acad Dermatol. 2018;78(1):141–147. doi: 10.1016/j.jaad.2017.07.031. [DOI] [PubMed] [Google Scholar]
- Bay C., Lerche C.M., Ferrick B., Philipsen P.A., Togverd-Bo K., Haedersdal M. Comparison of physical pretreatment regimens to enhance protoporphyrin IX uptake in photodynamic therapy: A randomized clinical trial. JAMA Dermatol. 2017;153(4):270–278. doi: 10.1001/jamadermatol.2016.5268. [DOI] [PubMed] [Google Scholar]
- Becker L.R., Aakhus A.E., Reich H.C., Lee P.K. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153(4):321–322. doi: 10.1001/jamadermatol.2016.5058. [DOI] [PubMed] [Google Scholar]
- Benson E. Imiquimod: Potential risk of an immunostimulant. Australas J Dermatol. 2004;45(2):123–124. doi: 10.1111/j.1440-0960.2004.00060.x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A., Patel R., Werschler W.P., Ceilley R.I., Strimling R. High-dose rate electronic brachytherapy: A nonsurgical treatment alternative for nonmelanoma skin cancer. J Clin Aesthet Dermatol. 2016;9(11):16–22. [PMC free article] [PubMed] [Google Scholar]
- Bichakjian C., Olencki T., Aasi S., Alam M., Anderson J.S., Blitzblau R. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN Guidelines®) basal cell skin cancer, version 1.2019 [Internet] 2017. http://www.NCCN.org [cited March 29,2019]. Available from.
- Bichakjian C., Olencki T., Aasi S., Alam M., Anderson J.S., Blitzblau R. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN Guidelines®) squamous cell skin cancer, version 2.2019 [Internet] 2017. http://www.NCCN.org [cited March 29, 2019]. Available from.
- Blum V., Müller B., Hofer S., Pardo E., Zeidler K., Diebold J. Nivolumab for recurrent cutaneous squamous cell carcinoma: three cases. Eur J Dermatol. 2018;28(1):78–81. doi: 10.1684/ejd.2017.3209. [DOI] [PubMed] [Google Scholar]
- Bolotin D., Alam M. Surgery of the Skin. 3rd ed. Elsevier; New York: 2015. Electrosurgery; pp. 134–149. [Google Scholar]
- Braathen L.R., Szeimies R.M., Basset-Seguin N., Bissonnette R., Foley P., Pariser D. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: An international consensus. J Am Acad Dermatol. 2007;56(1):125–143. doi: 10.1016/j.jaad.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Bray F.N., Simmons B.J., Wolfson A.H., Nouri K. Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatol Ther (Heidelb) 2016;6(2):185–206. doi: 10.1007/s13555-016-0120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brin L., Zubair A.S., Brewer J.D. Optimal management of skin cancer in immunosuppressed patients. Am J Clin Dermatol. 2014;15(4):339–356. doi: 10.1007/s40257-014-0085-5. [DOI] [PubMed] [Google Scholar]
- Bubna A.K. Imiquimod – Its role in the treatment of cutaneous malignancies. Indian J Pharmacol. 2015;47(4):354–359. doi: 10.4103/0253-7613.161249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunick C.G., Aasi S.Z. Hemorrhagic complications in dermatologic surgery. Dermatol Ther. 2011;24(6):537–550. doi: 10.1111/j.1529-8019.2012.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan S., Goldsberry K., Kim G., Yoo S. The management of antithrombotic medication in skin surgery. Dermatol Surg. 2012;38(9):1417–1426. doi: 10.1111/j.1524-4725.2012.02490.x. [DOI] [PubMed] [Google Scholar]
- Chitwood K., Etzkorn J., Cohen G. Topical and intralesional treatment of nonmelanoma skin cancer: Efficacy and cost comparisons. Dermatol Surg. 2013;39(9):1306–1316. doi: 10.1111/dsu.12300. [DOI] [PubMed] [Google Scholar]
- Chren M.M., Sahay A.P., Bertenthal D.S., Sen S., Landefeld C.S. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351–1357. doi: 10.1038/sj.jid.5700740. [DOI] [PubMed] [Google Scholar]
- Chren M.M., Linos E., Torres J.S., Stuart S.E., Parvataneni R., Boscardin W.J. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133(5):1188–1196. doi: 10.1038/jid.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayman G.L., Lee J.J., Holsinger F.C., Zhou X., Duvic M., El-Naggar A.K. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23(4):759–765. doi: 10.1200/JCO.2005.02.155. [DOI] [PubMed] [Google Scholar]
- Cognetta A.B., Jr., Wolfe C.M., Goldberg D.J., Hong H.G. Practice and educational gaps in radiation therapy in dermatology. Dermatol Clin. 2016;34(3):319–333. doi: 10.1016/j.det.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Cohen D.K., Lee P.K. Photodynamic therapy for non-melanoma skin cancers. Cancers (Basel) 2016;8(10):90. doi: 10.3390/cancers8100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.L., Perone J.B. A prospective evaluation of the incidence of complications associated with Mohs micrographic surgery. Arch Dermatol. 2003;139(2):143–152. doi: 10.1001/archderm.139.2.143. [DOI] [PubMed] [Google Scholar]
- Cortés-Peralta E.C., Ocampo-Candiani J., Vázquez-Martínez O.T., Gutiérrez-Villarreal I.M., Miranda-Maldonado I., Garza-Rodríguez V. Correlation between incisional biopsy histological subtype and a Mohs surgery specimen for nonmelanoma skin cancer. Actas Dermosifiliogr. 2018;109(1):47–51. doi: 10.1016/j.ad.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Cumberland L., Dana A., Liegeois N. Mohs micrographic surgery for the management of nonmelanoma skin cancers. Facial Plast Surg Clin North Am. 2009;17(3):325–335. doi: 10.1016/j.fsc.2009.06.001. [DOI] [PubMed] [Google Scholar]
- David C.V. Electronic brachytherapy and superficial radiation therapy: Will you be adding it to your practice? Cutis. 2013;92(5):E16–E18. [PubMed] [Google Scholar]
- Delishaj D., Rembielak A., Manfredi B., Ursino S., Pasqualetta F., Laliscia C. Non-melanoma skin cancer treated with high-dose-rate brachytherapy: A review of literature. J Contemp Brachytherapy. 2016;8(6):533–540. doi: 10.5114/jcb.2016.64112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffey B.L., Langtry J.A. Skin cancer incidence and the ageing population. Br J Dermatol. 2005;153(3):679–680. doi: 10.1111/j.1365-2133.2005.06799.x. [DOI] [PubMed] [Google Scholar]
- Divine J., Stefaniwksy L., Reddy R., Padilla P., Hagele T., Patel N.S. A comprehensive guide to the surgical management of nonmelanoma skin cancer. Curr Probl Cancer. 2015;39(4):216–225. doi: 10.1016/j.currproblcancer.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Dréno B., Kunstfeld R., Hauschild A., Fosko S., Zloty D., Labeille B. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): A randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18(3):404–412. doi: 10.1016/S1470-2045(17)30072-4. [DOI] [PubMed] [Google Scholar]
- Fellner C. Vismodegib (erivedge) for advanced Basal cell carcinoma. P T. 2012;37(12):670–682. [PMC free article] [PubMed] [Google Scholar]
- Fink C., Enk A., Gholam P. Photodynamic therapy—Aspects of pain management. J Dtsch Dermatol Ges. 2015;13(1):15–22. doi: 10.1111/ddg.12546. [DOI] [PubMed] [Google Scholar]
- Fox M., Helfrich Y., Kang S. Other topical medications. In: Bolognia J., Schaffer J., Cerroni L., editors. Dermatology. 4th ed. Elsevier; Philadelphia: 2018. pp. 2263–2277. [Google Scholar]
- Galles E., Parvataneni R., Stuart S.E., Linos E., Grewal S., Chren M.M. Patient-reported outcomes of electrodessication & curettage for treatment of non-melanoma skin cancer. J Am Acad Dermatol. 2014;71(5):1026–1028. doi: 10.1016/j.jaad.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcovich S., Colloca G., Sollena P., Bellieni A., Balducci L., Cho W. Cancer epidemics in the elderly as an emerging issue in geriatric oncology. Aging Dis. 2017;8(5):643–661. doi: 10.14336/AD.2017.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisse J., Caro I., Lindholm J., Golitz L., Stampone P., Owens M. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studies. J Am Acad Dermatol. 2004;50(5):722–733. doi: 10.1016/j.jaad.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Gerritsen M.J., Smits T., Kleinpenning M.M., van de Kerkhof P.C., van Erp P.E. Pretreatment to enhance protoporphyrin IX accumulation in photodynamic therapy. Dermatology. 2009;218(3):193–202. doi: 10.1159/000183753. [DOI] [PubMed] [Google Scholar]
- Giordano Resti A., Sacconi R., Baccelli N., Bendello F. Outcome of 110 basal cell carcinomas of the eyelid treated with frozen section-controlled excision: Mean follow-up over 5 years. Eur J Ophthalmol. 2014;24(4):476–482. doi: 10.5301/ejo.5000405. [DOI] [PubMed] [Google Scholar]
- Glass J.S., Hardy C.L., Meeks N.M., Carroll B.T. Acute pain management in dermatology: Risk assessment and treatment. J Am Acad Dermatol. 2015;73(4):543–560. doi: 10.1016/j.jaad.2015.04.050. [DOI] [PubMed] [Google Scholar]
- Grant-Kels J.M., VanBeek M.J. The ethical implications of "more than one way to skin a cat": Increasing use of radiation therapy to treat nonmelanoma skin cancers by dermatologists. J Am Acad Dermatol. 2014;70(5):945–947. doi: 10.1016/j.jaad.2014.01.849. [DOI] [PubMed] [Google Scholar]
- Guo S., DiPietro L.A. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulyalkar R., Rakkhit T., Garcia-Zuazaga J. The role of radiation therapy in the management of skin cancers. Dermatol Clin. 2011;29(2):287–296. doi: 10.1016/j.det.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Hussain W., Affleck A., Al-Niaimi F., Cooper A., Craythorne E., Fleming C. Safety, complications and patients' acceptance of Mohs micrographic surgery under local anaesthesia: Results from the U.K. MAPS (Mohs Acceptance and Patient Safety) Collaboration Group. Br J Dermatol. 2017;176(3):806–808. doi: 10.1111/bjd.14843. [DOI] [PubMed] [Google Scholar]
- Izikson L., Seyler M., Zeitouni N. Prevalence of Underdiagnosed Aggressive Non-Melanoma Skin Cancers Treated with Mohs Micrographic Surgery: Analysis of 513 Cases. Dermatol Surg. 2010;36:1769–1772. doi: 10.1111/j.1524-4725.2010.01747.x. [DOI] [PubMed] [Google Scholar]
- Jarkowski A., 3rd, Hare R., Loud P., Skitzki J.J., Kane J.M., 3rd, May K.S. Systemic therapy in advanced cutaneous squamous cell carcinoma (CSCC): The Roswell Park Experience and a review of the literature. Am J Clin Oncol. 2016;39(6):545–548. doi: 10.1097/COC.0000000000000088. [DOI] [PubMed] [Google Scholar]
- Jung J.Y., Linos E. Adding active surveillance as a treatment option for low risk cancers in patients with limited life expectancy. J Geriatr Oncol. 2016;7(3):221–222. doi: 10.1016/j.jgo.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Karia P.S., Han J., Schmults C.D. Cutaneous squamous cell carcinoma: Estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957–966. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- Kauvar A.N., Cronin T., Jr., Roenigk R., Hruza G., Bennett R. Consensus for nonmelanoma skin cancer treatment: Basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550–571. doi: 10.1097/DSS.0000000000000296. [DOI] [PubMed] [Google Scholar]
- Kauvar A.N., Arpey C.J., Hruza G., Olbricht S.M., Bennett R., Mahmoud B.H. Consensus for nonmelanoma skin cancer treatment, part II: Squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214–1240. doi: 10.1097/DSS.0000000000000478. [DOI] [PubMed] [Google Scholar]
- Kohler-Brock A., Prager W., Pohlmann S., Kunze S. The indications for and results of HDR afterloading therapy in diseases of the skin and mucosa with standardized surface applicators (the Leipzig applicator) Strahlenther Onkol. 1999;175(4):170–174. [PubMed] [Google Scholar]
- Lehmann P. Side effects of topical photodynamic therapy. Hautarzt. 2007;58(7):597–603. doi: 10.1007/s00105-007-1363-4. [DOI] [PubMed] [Google Scholar]
- Leibovitch I., Huilgol S.C., Selva D., Hill D., Richards S., Paver R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia I: Experience over 10 years. J Am Acad Dermatol. 2005;53(2):253–260. doi: 10.1016/j.jaad.2005.02.059. [DOI] [PubMed] [Google Scholar]
- Lien M.H., Sondak V.K. Nonsurgical treatment options for basal cell carcinoma. J Skin Cancer. 2011;2011 doi: 10.1155/2011/571734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linos E. Treatment of nonfatal conditions at the end of life: Nonmelanoma skin cancer. JAMA Intern Med. 2013;173:1006–1012. doi: 10.1001/jamainternmed.2013.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke J., Karimpour S., Young G., Lockett M.A., Perez C.A. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):748–755. doi: 10.1016/s0360-3016(01)01656-x. [DOI] [PubMed] [Google Scholar]
- Love W.E., Bernhard J.D., Bordeaux J.S. Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: A systematic review. Arch Dermatol. 2009;145(12):1431–1438. doi: 10.1001/archdermatol.2009.291. [DOI] [PubMed] [Google Scholar]
- Lyons A.B., Chipps L.K., Moy R.L., Herrmann J.L. Dehydrated human amnion/chorion membrane allograft as an aid for wound healing in patients with full-thickness scalp defects after Mohs micrographic surgery. JAAD Case Rep. 2018;4(7):688–691. doi: 10.1016/j.jdcr.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie-Wood A., Kossard S., de Launey J., Wilkinson B., Owens M.L. Imiquimod 5% cream in the treatment of Bowen's disease. J Am Acad Dermatol. 2001;44(3):462–470. doi: 10.1067/mjd.2001.111335. [DOI] [PubMed] [Google Scholar]
- Marzuka A.G., Book S.E. Basal cell carcinoma: Pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88(2):167–179. [PMC free article] [PubMed] [Google Scholar]
- Matei C., Tampa M., Poteca T., Panea-Paunica G., Georgescu S.R., Ion R.M. Photodynamic therapy in the treatment of basal cell carcinoma. J Med Life. 2013;6(1):50–54. [PMC free article] [PubMed] [Google Scholar]
- Mendenhall W.M., Ferlito A., Takes R.P., Bradford C.R., Corry J., Fagan J.J. Cutaneous head and neck basal and squamous cell carcinomas with perineural invasion. Oral Oncol. 2012;48(10):918–922. doi: 10.1016/j.oraloncology.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Micali G., Lacarrubba F., Nascaa Ferraro S., Schwartz R. Topical pharmacotherapy for skin cancer Part II. Clinical applications. J Am Acad Dermatol. 2014;70(6):979.e1-12. doi: 10.1016/j.jaad.2013.12.037. [DOI] [PubMed] [Google Scholar]
- Muzic J.G., Schmitt A.R., Wright A.C., Alniemi D.T., Zubair A.S., Olazagasti Loundo J.M. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: A population-based study in Olmsted County, Minnesota, 2000 to 2019. Mayo Clin Proc. 2017;92(6):890–898. doi: 10.1016/j.mayocp.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville J.A., Welch E., Leffell D.J. Management of nonmelanoma skin cancer in 2007. Nat Clin Pract Oncol. 2007;4(8):462–469. doi: 10.1038/ncponc0883. [DOI] [PubMed] [Google Scholar]
- Olek D., Jr., El-Ghamry M.N., Deb N., Thawani N., Shaver C., Mutyala S. Custom mold applicator high-dose-rate brachytherapy for nonmelanoma skin cancer-An analysis of 273 lesions. Brachytherapy. 2018;17(3):601–608. doi: 10.1016/j.brachy.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Ozog D.M., Rkein A.M., Fabi S.G., Gold M.H., Goldman M.P., Lowe N.J. Photodynamic therapy: A clinical consensus guide. Dermatol Surg. 2016;42(7):804–827. doi: 10.1097/DSS.0000000000000800. [DOI] [PubMed] [Google Scholar]
- Parikh S.A., Patel V.A., Ratner D. Advances in the management of cutaneous squamous cell carcinoma. F1000Prime Rep. 2014;6:70. doi: 10.12703/P6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.K., Hagstrom E., Berrios R., Tung R.C. A novel purse-string suture and dehydrated human amnion/chorion membrane allograft closure technique for the repair of defects following Mohs micrographic and excisional surgery. J Clin Investigat Dermatol. 2016;4(1):1–3. [Google Scholar]
- Patel R., Strimling R., Doggett S., Willoughby M., Miller K., Dardick L. Comparison of electronic brachytherapy and Mohs micrographic surgery for the treatment of early-stage non-melanoma skin cancer: A matched pair cohort study. J Contemp Brachytherapy. 2017;9(4):338–344. doi: 10.5114/jcb.2017.68480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris K., Micantonio T., Fargnoli M.C., Lozzi G.P., Chimenti S. Imiquimod 5% cream in the treatment of Bowen's disease and invasive squamous cell carcinoma. J Am Acad Dermatol. 2006;55(2):324–327. doi: 10.1016/j.jaad.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Prickett K.A., Ramsey L. Mohs micrographic surgery. [Updated 2017 Oct 6]. In: StatPearls [Internet] 2017. https://www.ncbi.nlm.nih.gov/books/NBK441833/ [cited January 3, 2019]. Available from.
- Puig S., Berrocal A. Management of high-risk and advanced basal cell carcinoma. Clin Transl Oncol. 2015;17(7):497–503. doi: 10.1007/s12094-014-1272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk C., Gebauer K., De'Ambrosis B., Slade H.B., Meng T.C. Sustained clearance of superficial basal cell carcinomas treated with imiquimod cream 5%: Results of a prospective 5-year study. Cutis. 2010;85(6):318–324. [PubMed] [Google Scholar]
- Raasch B. Management of superficial basal cell carcinoma: Focus on imiquimod. Clin Cosmet Investig Dermatol. 2009;2:65–75. doi: 10.2147/ccid.s3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiger K.E., Linos E., Egbert B.M., Swetter S.M. Recurrence rates associated with incompletely excised low-risk nonmelanoma skin cancer. J Cutan Pathol. 2010;37:59–67. doi: 10.1111/j.1600-0560.2009.01340.x. [DOI] [PubMed] [Google Scholar]
- Rkein A.M., Ozog D.M. Photodynamic therapy. Dermatol Clin. 2014;32(3):415–425. doi: 10.1016/j.det.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Rogers H.W., Weinstock M.A., Feldman S.R., Coldiron B.M. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151(10):1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- Rosen T., Harting M., Gibson M. Treatment of Bowen's disease with topical 5% imiquimod cream: Retrospective study. Dermatol Surg. 2007;33(4):427–432. doi: 10.1111/j.1524-4725.2007.33089.x. [DOI] [PubMed] [Google Scholar]
- Rowe D.E., Carroll R.J., Day C.L., Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992;26(6):976–990. doi: 10.1016/0190-9622(92)70144-5. [DOI] [PubMed] [Google Scholar]
- Saad S., Mansouri B., Housewright C. Acute exacerbation of myasthenia gravis with topical imiquimod use. Proc (Bayl Univ Med Cent) 2017;30(3):333. doi: 10.1080/08998280.2017.11929637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarasinghe V., Madan V. Nonmelanoma skin cancer. J Cutan Aesthet Surg. 2012;5(1):3–10. doi: 10.4103/0974-2077.94323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savant S., Savant S., Sehgal V.N. Cryosurgery for facial and scalp lesions of basal cell carcinoma: A study in 29 elderly patients. Skinmed. 2017;15(5):357–364. [PubMed] [Google Scholar]
- Savoia P., Deboli T., Previgliano A., Broganelli P. Usefulness of photodynamic therapy as a possible therapeutic alternative in the treatment of basal cell carcinoma. Int J Mol Sci. 2015;16(10):23300–23317. doi: 10.3390/ijms161023300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton M., Jones D.B., Maloney M.E. Histologic pattern analysis of basal cell carcinoma. Study of a series of 1039 consecutive neoplasms. J Am Acad Dermatol. 1990;23(6 Pt 1):1118–1126. doi: 10.1016/0190-9622(90)70344-h. [DOI] [PubMed] [Google Scholar]
- Sheridan A.T., Dawber R.P. Curettage, electrosurgery and skin cancer. Australian J Dermatol. 2000;41(1):19–30. doi: 10.1046/j.1440-0960.2000.00383.x. [DOI] [PubMed] [Google Scholar]
- Shriner D.L., McCoy D.K., Goldberg D.J., Wagner R.F., Jr. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39(1):79–97. doi: 10.1016/s0190-9622(98)70405-0. [DOI] [PubMed] [Google Scholar]
- Smeets N.W., Kuijpers D.I., Nelemans P., Ostertag J.U., Verhaegh M.E., Krekels G.A. Mohs' micrographic surgery for treatment of basal cell carcinoma of the face--Results of a retrospective study and review of the literature. Br J Dermatol. 2004;151(1):141–147. doi: 10.1111/j.1365-2133.2004.06047.x. [DOI] [PubMed] [Google Scholar]
- Smucler R., Vlk M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg Med. 2008;40(2):153–158. doi: 10.1002/lsm.20606. [DOI] [PubMed] [Google Scholar]
- Souza C.S., Neves A.B., Felício L.A., Ferreira J., Kurachi C., Bagnato V.S. Optimized photodynamic therapy with systemic photosensitizer following debulking technique for nonmelanoma skin cancers. Dermatol Surg. 2007;33(2):194–198. doi: 10.1111/j.1524-4725.2006.33038.x. [DOI] [PubMed] [Google Scholar]
- Soyer H.P., Rigel D., McMeniman E. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Bolognia J., Schaffer J., Cerroni L., editors. Dermatology. 4th ed. Elsevier; Philadelphia: 2018. pp. 1872–1893. [Google Scholar]
- Stanger M.J., Thompson L.A., Young A.J., Lieberman H.R. Anticoagulant activity of select dietary supplements. Nutr Rev. 2012;70(2):107–117. doi: 10.1111/j.1753-4887.2011.00444.x. [DOI] [PubMed] [Google Scholar]
- Steinbauer J.M., Schreml S., Kohl E.A., Karrer S., Landthaler M., Szeimies R.M. Photodynamic therapy in dermatology. J Dtsch Dematol Ges. 2010;8(6):454–464. doi: 10.1111/j.1610-0387.2010.07343.x. [DOI] [PubMed] [Google Scholar]
- Thompson A.K., Kelley B.F., Prokop L.J., Murad M.H., Baum C.L. Risk factors for cutaneous squamous cell carcinoma outcomes: A systemic review and meta-analysis. JAMA Dermatol. 2016;152(4):419–428. doi: 10.1001/jamadermatol.2015.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Celada F., Rodriguez S., Botella R., Ballesta A., Kasper M. Non-melanoma skin cancer treated with HDR Valencia applicator: Clinical outcomes. J Contemp Brachytherapy. 2014;6(2):167–172. doi: 10.5114/jcb.2014.43247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D.C., Colevas A.D., Chang A.L. Follow-up on programmed cell death 1 inhibitor for cutaneous squamous cell carcinoma. JAMA Dermatol. 2017;153(1):92–94. doi: 10.1001/jamadermatol.2016.3884. [DOI] [PubMed] [Google Scholar]
- Vuong W., Lin J., Wei R.L. Palliative radiotherapy for skin malignancies. Ann Palliat Med. 2017;6(2):165–172. doi: 10.21037/apm.2016.11.10. [DOI] [PubMed] [Google Scholar]
- Warshauer E., Warshauer B.L. Clearance of basal cell and superficial squamous cell carcinomas after imiquimod therapy. J Drugs Dermatol. 2008;7(5):447–451. [PubMed] [Google Scholar]
- Wennberg A.M., Larkö O., Stenquist B. Five-year results of Mohs micrographic surgery for aggressive facial basal cell carcinoma in Sweden. Acta Derm Venereol. 1999;79(5):370–372. doi: 10.1080/000155599750010292. [DOI] [PubMed] [Google Scholar]
- Whitely L., Kuwahara R.T., Garcia C. Dermatologic surgery in the demented patient. Dermatol Surg. 2003;29(3):241–244. doi: 10.1046/j.1524-4725.2003.29057.x. [DOI] [PubMed] [Google Scholar]
- Wu J.K., Siller G., Strutton G. Psoriasis induced by topical imiquimod. Australas J Dermatol. 2004;45(1):47–50. doi: 10.1111/j.1440-0960.2004.00030.x. [DOI] [PubMed] [Google Scholar]
- Yakish K., Graham J., Hossler E.W. Efficacy of curettage alone for invasive cutaneous squamous cell carcinoma: A retrospective cohort study. J Am Acad Dermatol. 2017;77(3):582–584. doi: 10.1016/j.jaad.2017.04.1108. [DOI] [PubMed] [Google Scholar]
- Yang X., Dinehart S.M. Intermittent vismodegib therapy in basal cell nevus syndrome. JAMA Dermatol. 2016;152(2):223–224. doi: 10.1001/jamadermatol.2015.3210. [DOI] [PubMed] [Google Scholar]
- Zouboulis C.C. Cryosurgery in dermatology. Hautarzt. 2015;66(11):834–848. doi: 10.1007/s00105-015-3703-0. [DOI] [PubMed] [Google Scholar]