Abstract
Introduction
Artemisinin-based combination therapy (ACT) is recommended by the World Health Organization as first-line treatment of uncomplicated Plasmodium falciparum malaria. ACT treatments failures among travellers returning from Africa to non-endemic countries are considered to be caused by resistance.
Case presentation
We report on a case of artemether-lumefantrine treatment failure in a Turkish traveller with uncomplicated P. falciparum malaria returning from Bamako, Mali.
Conclusions
Information on returning travellers, includes ensuring that the patients receive supervised treatment with the recommended dose of a quality controlled medicine, routine follow-up of all cases, assessment of adequate absorption of the drug, and/or testing the prevalence of molecular markers of drug resistance if validated, can be an important source of an early warning system for emerging resistance.
Keywords: Malaria, P. falciparum, Artemisinin-based combination therapy
Introduction
Turkey has succeeded in eliminating malaria with the last indigenous Plasmodium vivax case reported in 2009, and the last Plasmodium falciparum case reported in 1969 [1,2]. In 2016, Turkey had 209 cases of imported malaria [3]. Uncomplicated falciparum patients are treated with the ACT artemether-lumefantrine (AL).
Countries that have eliminated malaria need retain a strong surveillance systems to quickly detect cases, and skilled personnel to diagnose and treat them. This will both help prevent reintroduction of malaria, and ensure that imported cases get the treatment needed [4].
Too often treatment failures are considered to be caused by resistance and the case presented below is a good example of this. We report on a case of AL treatment failure in a Turkish traveller with uncomplicated P. falciparum malaria returning from Bamako, Mali.
Case report
A 42 year old Turkish man was admitted 20 September 2017 to the Department of Emergency in Gulhane Training and Research Hospital, Ankara, Turkey with fever, headache and vomiting for the past ten days. He had returned from one month’s business travel to Bamako, Mali, 27 days prior to admission. He had used mefloquine chemoprophylaxis against malaria before travelling but reported not taking his medications regularly. The living conditions during the stay in Mali were reported to be poor with many mosquitoes present. Upon admission, the patient’s body temperature was 38.3 °C, his pulse rate was 90 beats/min, and his blood pressure was 110/75 mmHg. Laboratory test results showed low haemoglobin level of 12.3 g/L, a platelet count of 52 × 103 cells/μl, a leukocyte count of 3.91 × 103cells/ μl, a red blood cell count of 3,96 × 103 cells/μl (Table 1). Abdominal ultrasonography showed increase in liver size (170 mm) and spleen size (138 mm) of the patient. Cultures from blood, urine and stool taken at and during admission were all negative. A peripheral blood smear was performed and was reported to be positive for P. vivax but a second reading identified P. falciparum and P. vivax mixed infection. Consequently, the patient was admitted to the Department of Infectious Diseases. Nested-PCR was performed and found the patient positive for P. falciparum only. As a result, the following treatment was administered under supervision: artemether-lumefantrine (20 mg/120 mg Riamet®; 4 tablets per dose at 0, 8, 24, 36, 48 and 60 h) and primaquine (30 mg per day for 14 days). As recommended by WHO, the first control blood smear was taken at day 2 (48 h after beginning of the AL treatment). Only gametocyte forms of the parasite and no asexual forms were observed (Fig. 1). On day 14 of the treatment, the patient was afebrile and blood microscopy was negative for P. falciparum and he was discharged 2 days later.
Table 1.
Blood results of the patient.
| Variable | Admission 1 First day | Admission 1 14th day | Admission 2 First day | Admission 2 14th day | Reference value |
|---|---|---|---|---|---|
| Leukocytes | 3.91 | 6.79 | 4.59 | 5.74 | 3.91-10.9 × 103cells/ μl |
| Erytrocytes | 3.96 | 3.93 | 3.78 | 3.31 | 4.44-5.61 × 103cells/ μl |
| Hemoglobin | 12.3 | 12.1 | 11.9 | 9.8 | 13.5-16.9 g/L |
| Hematocrit | 33.3 | 35.6 | 33.1 | 28.2 | 40-49.4 % |
| Platelets | 52 | 397 | 85 | 289 | 173-360 × 103 cells/μl |
| ASTa | 53 | 34 | 30 | 26 | 15-40 U/L |
| ALTb | 26 | 35 | 17 | 15 | 10-40 U/L |
| LDHc | 392 | – | – | – | >0-<248 U/L |
| Amilase | 27 | – | – | – | >28-<100 U/L |
| Total bilirubin | 1.6 | 1 | 1.3 | 0.6 | >0.3-<1.2 mg/dl |
| Direct bilirubin | 0.32 | 0.2 | 0.25 | 0.08 | 0-0.2 mg/dl |
| Albumin | 3.2 | 3.9 | 3.5 | 3.7 | 3.5-5.2 g/dl |
| Creatinine | 1.49 | 0.98 | 0.94 | 1.02 | 0.84-1.25 mg/dl |
| Urea | 54 | 25 | 33 | 21 | 17-43 mg/dl |
| Uric acid | 7.5 | – | – | 7.2 | >3.5-<7.2 mg/dl |
| C-reactive protein | 169,61 | 4.71 | 89.69 | 20.19 | 0-5 mg/L |
| Sedimentation rate | 68 | 71 | 75 | 84 | 0-20 mm/h |
AST: Aspartate transaminase.
ALT: Alanine transaminase.
LDH: Lactate dehydrogenase.
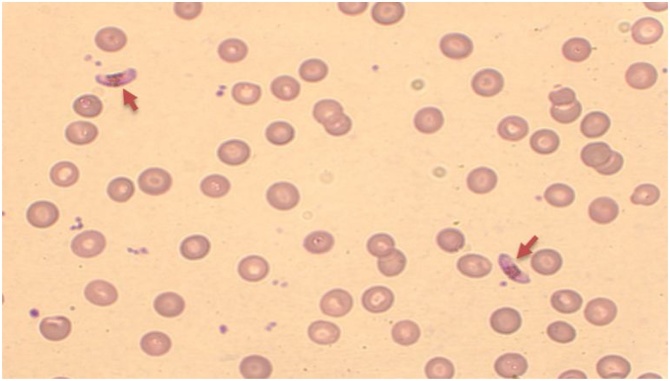
Fig. 1.
The first control blood smear of the patient with only gametocyte forms of the parasite (arrowheads) (magnification ×1000).
The patient was readmitted to the Department of Infectious Diseases 35 days after the initial admission with a body temperature at 37.8 °C, sweating, dysuria, diarrhoea, nausea and vomiting. Blood test results are summarized in Table 1. Cultures from blood, urine and stool were all negative. Sera from the patient was negative for Brucella and Salmonella antigens by ELISA and agglutination tests. Blood smear was examined and found positive for P. falciparum with 0.2% parasitemia and presence of sexual stages. Real-time PCR was performed and confirmed the diagnosis of P. falciparum. The patient reported that he had vomited soon after the initial medication, without having informed the clinic nurses and doctors of this. A treatment of artemether-lumefantrine was restarted. Half an hour before the treatment, the patient was given metoclopramide by slow intravenous injection as antiemetic and was followed up closely to detect any vomiting throughout the treatment. After 9 days, the patient was discharged in good clinical condition and with blood smear found to be negative for P. falciparum. The patient did not report further febrile episodes.
Discussion
We have reported on a case of Turkish traveller returning from Mali infected with P. falciparum malaria, and failing treatment after the administration of a WHO recommended ACT AL. Recent reports of such ACT treatments failures among travellers returning from Africa to non-endemic countries have led to pronouncement of the appearance of resistance to artemisinins and ACT partner drugs in Africa despite the authors of these reports clearly stating that there was not enough evidence to confirm resistance [5,6]. As this case report shows, such assertions can easily be done prematurely. Information on drug blood levels, in vitro susceptibility, or molecular markers for drug resistance were not available. In addition, the patient is reported to have vomited after treatment. The most likely cause of treatment failure is inadequate lumefantrine exposure as it happens frequently with AL when not taken with fatty food. In this case poor exposure could be associated or not with a single vomiting as the patient was finally cured with the same treatment. Artemether is not subject to such variability and therefore was able to reduce biomass appropriately.
As seen in the past, returning travellers such as the case reported on here could be an important source of information [7]. Data from travellers could serve as a warning system that could both supplement data collected in endemic countries as well as inform where therapeutic efficacy studies urgently need to be conducted. However, for this to be the case, information on returning travellers needs to be collected and reported systematically. This includes ensuring that the patients receive supervised treatment with the recommended dose of a quality controlled medicine, routine follow-up of all cases, assessment of adequate absorption of the drug, and/or testing the prevalence of molecular markers of drug resistance if validated. Collecting such detailed information for all returning travellers with malaria is not likely to be feasible but it should be possible to do so at central hospitals receiving a relatively large number of malaria patients.
A challenge especially in facilities that only see few cases of malaria is that microscopic examination of blood smears can sometimes fail to correctly identify the infecting species, especially in samples with low parasitemia or in mixed infections. In such cases, a molecular PCR test can be required to confirm microscopy results or resolve suspicious cases. Nested PCR is more sensitive compared to microscopic examination of Giemsa-stained thick and thin blood smears, particularly the detection of Plasmodium in cases with low parasitemia and mixed infection of malaria as shown by various studies [8,9].
In conclusion, resistance is just one of the possible causes of a malaria treatment failure. It is important that malaria diagnostic capabilities are maintained in countries also after malaria is eliminated. Information collected from travellers can be an important source of an early warning system for emerging resistance, provided the data collected are reliable and complete.
Author agreement
The Author hereby grants the Journal IDCases full and exclusive rights to the manuscript, all revisions, and the full copyright. The Journal IDCases rights include but are not limited to the following: (1) to reproduce, publish, sell, and distribute copies of the manuscript, selections of the manuscript, and translations and other derivative works based upon the manuscript, in print, audio-visual, electronic, or by any and all media now or hereafter known or devised; (2) to license reprints of the manuscript to third persons for educational photocopying; (3) to license others to create abstracts of the manuscript and to index the manuscript; (4) to license secondary publishers to reproduce the manuscript in print, microform, or any computer-readable form, including electronic on-line databases; and (5) to license the manuscript for document delivery. These exclusive rights run the full term of the copyright, and all renewals and extensions thereof. I hereby accept the terms of the above Author Agreement and I sign on behalf of the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authorship verification
All co-authors have seen and agree with the contents of the manuscript and have contributed significantly to the work.
Disclaimer
CR and PR are staff member of the World Health Organization. Only CR and PR are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the World Health Organization.
Declaration of Competing Interest
None of the authors report any conflicts of interest.
References
- 1.World Health Organization . University of California; San Francisco: 2013. Eliminating malaria: case study 5. The long road to malaria elimination in Turkey. [Google Scholar]
- 2.Piyal B., Akdur R., Ocaktan E., Yozgatligil C. An analysis of the prevalence of malaria in Turkey over the last 85 years. Pathog Glob Health. 2013;107(1):30–34. doi: 10.1179/2047773212Y.0000000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . World Health Organization; Geneva: 2017. World malaria report 2017. [Google Scholar]
- 4.Dharmawardena P., Rodrigo C., Mendis K., de A.W., Gunasekera W.M.K.T., Premaratne R. Response of imported malaria patients to antimalarial medicines in Sri Lanka following malaria elimination. PLoS One. 2017;12(11):e0188613. doi: 10.1371/journal.pone.0188613. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutherland C.J., Lansdell P., Sanders M., Muwanguzi J., van Schalkwyk D.A., Kaur H. Pfk13-independent treatment failure in four imported cases of Plasmodium falciparum malaria treated with artemether-lumefantrine in the United Kingdom. Antimicrob Agents Chemother. 2017;61(3):e02382–16. doi: 10.1128/AAC.02382-16. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sondén K., Wyss K., Jovel I., Vieira da Silva A., Pohanka A., Asghar M. High rate of treatment failures in nonimmune travelers treated with artemether-lumefantrine for uncomplicated Plasmodium falciparum malaria in Sweden: retrospective comparative analysis of effectiveness and case series. Clin Infect Dis. 2017;64(2):199–206. doi: 10.1093/cid/ciw710. 15. [DOI] [PubMed] [Google Scholar]
- 7.Basco L.K., Ringwald P., Simon F., Doury J.C., Lebras J. Evolution of chloroquine resistance in Central and West Africa. Trop Med Parasitol. 1993;44:111–112. [PubMed] [Google Scholar]
- 8.Johnston S.P., Pieniazek N.J., Xayavong M.V., Slemende S.B., Wilkins P.P., da Silva A.J. PCR as a confirmatory Technique for laboratory diagnosis of malaria. J Clin Microbiol. 2006;44:1087–1089. doi: 10.1128/JCM.44.3.1087-1089.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanscheid T. Current strategies to avoid misdiagnosis of malaria. Clin Microbiol Infect. 2003;9:497–504. doi: 10.1046/j.1469-0691.2003.00640.x. [DOI] [PubMed] [Google Scholar]