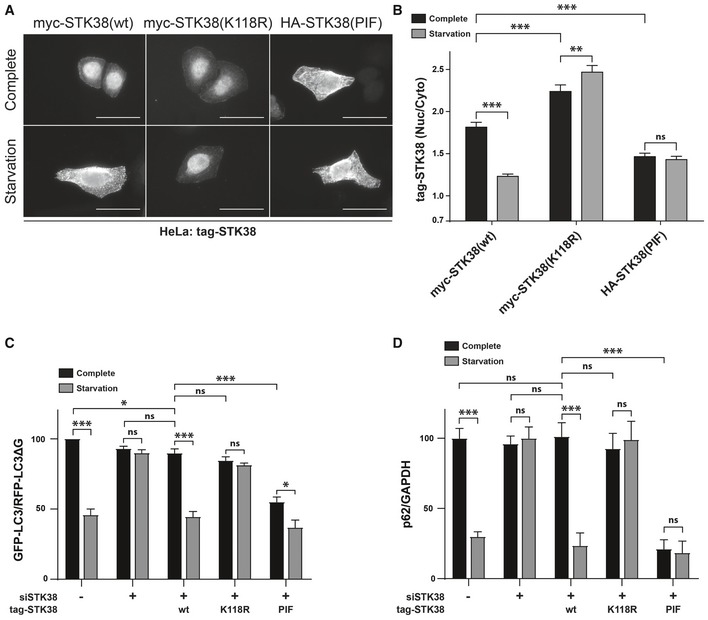
STK38 kinase activity is required and sufficient for nutrient starvation‐induced autophagy. (C) HeLa cells stably expressing the GFP‐LC3‐RFP‐LC3ΔG autophagic probe
26 were transiently transfected with siRNA targeting the 3′UTR region of endogenous STK38 (or with non‐targeting siRNA (siNT)). The next day, cells were transiently transfected with the indicated STK38 mutants expressing plasmids (identical to (A–B)). Twenty‐four hours after plasmid transfection, cells were incubated with DMEM or EBSS for 4 h. The GFP‐LC3 (degraded upon autophagy) and RFP‐LC3ΔG (non‐degraded upon autophagy) signals were recorded by FACS analysis and are shown as a ratio recapitulating overall LC3 level (
n = 4 independent experiments, mean ± SEM; *
P < 0.05, ***
P < 0.001, ns, not significant, Mann–Whitney test). As published
8, depleting STK38 prevents autophagy to take place. This effect was partially reversed by expressing the wt ORF as well as constitutively active STK38, whereas kinase‐dead version failed to reproduce endogenous STK38 effect. Expression of constitutively active STK38 is sufficient to induce a substantial change in the autophagic flux upon nutrient‐rich conditions (see
Appendix Fig S3A for STK38 replacement). (D) HeLa cells were transiently transfected with siRNA targeting the 3′UTR region of endogenous STK38 (or with non‐targeting siRNA (siNT)) and then transiently transfected with the indicated STK38 mutants expressing plasmids 48 h after. The next day, the cells were incubated with DMEM or EBSS for 4 h and overall p62 level was quantified for autophagy estimation (see
Appendix Fig S3B for blots) (
n = 3 independent experiments, mean ± SEM; ***
P <
0.001, ns, not significant, Student's
t‐test).