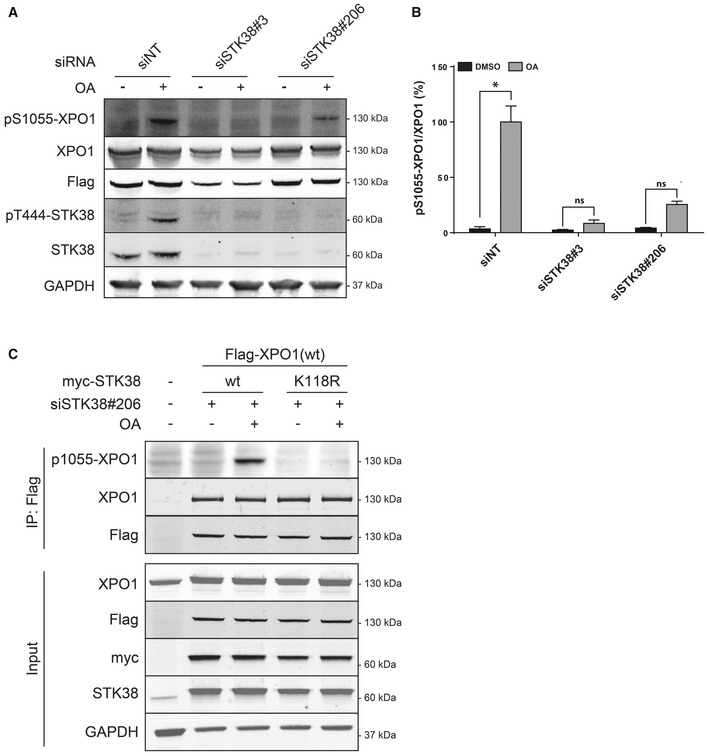
STK38 is required for XPO1_S1055 phosphorylation. (A) HeLa cells were transiently transfected with the indicated siRNA and subjected to Flag‐XPO1(wt) transient transfection the following day. Forty‐eight hours later, cells were incubated with okadaic acid (OA, final concentration = 1 μM) for 1 h or with DMSO. Immunoblotting was performed on whole‐cell lysates with the indicated antibodies. (B) Graphical representation of the phospho‐S1055‐XPO1 signal on total XPO1 (
n = 3 independent experiments, mean ± SEM; *
P <
0.05, ns, not significant, Mann–Whitney test). As expected, XPO1 is phosphorylated on its Ser1055 upon OA treatment but not when STK38 is silenced (see
Appendix Fig S5 for antibody validation and STK38 silencing quantification). (C) STK38 kinase activity is required for XPO1_S1055 phosphorylation. HekRasV12 cells silenced (or not) for endogenous STK38 were transfected with Flag‐XPO1(wt) expression plasmid in addition to myc‐STK38(wt) or myc‐STK38(K118R) (STK38 kinase‐dead version) plasmids. The next day, cells were treated with 1 μM OA or with vehicle (DMSO) for 1 h. Flag fusions were immunoprecipitated, and pulled‐down proteins were analyzed by Western blotting. The upper panel displays immunoprecipitated proteins, and the lower panel represents whole‐cell lysates.