Abstract
Objectives:
Despite recommendations for vaccination against hepatitis A virus (HAV) and hepatitis B virus (HBV) for all adults at increased risk of infection, several US states have reported increases in HAV and HBV infections among persons who inject drugs. We investigated hepatitis A and hepatitis B vaccination coverage among a sample of persons who reported injecting drugs and had evidence of hepatitis C virus (HCV) infection.
Methods:
We searched the Wisconsin Immunization Registry for the vaccination records of persons who underwent HCV testing at syringe services programs from January 1 through August 31, 2018, and were reported to the Wisconsin Division of Public Health as having positive HCV antibody test results and a history of injection drug use. We calculated the percentage of persons who were vaccinated according to national recommendations.
Results:
Of 215 persons reported, 204 (94.9%) had a client record in the Wisconsin Immunization Registry. Of these 204 persons, 66 (32.4%) had received ≥1 dose of hepatitis A vaccine, 46 (22.5%) had received 2 doses of hepatitis A vaccine, and 115 (56.4%) had received 3 doses of hepatitis B vaccine. Hepatitis B vaccine coverage decreased with increasing age, from 88.0% (22 of 25) among adults aged 20-24 to 30.3% (10 of 33) among adults aged 35-39.
Conclusions:
These findings suggest that most persons who inject drugs in Wisconsin are susceptible to HAV infection and that most persons aged ≥35 who inject drugs are susceptible to HBV infection. In addition to routine vaccination of children, targeted hepatitis vaccination programs should focus on adults who inject drugs to help prevent future infections.
Keywords: hepatitis A, hepatitis B, injection drug use, vaccination coverage, immunization information system
Viral hepatitis affects the health of millions of persons in the United States. The Centers for Disease Control and Prevention (CDC) estimated that, in 2016, 66 100 persons had an acute infection with viral hepatitis and more than 4 million persons had chronic viral hepatitis infection.1 Acute infection with hepatitis A virus (HAV), hepatitis B virus (HBV), or hepatitis C virus (HCV) can cause fever, nausea, vomiting, abdominal pain, and jaundice. Chronic infection with HBV or HCV can be asymptomatic for years but increases the risk of liver disease, liver cancer, and death. Persons who inject drugs are at increased risk for infection with viral hepatitis because HBV and HCV are blood-borne viruses that can remain infectious outside the body for weeks and can be easily transmitted through contaminated needles and other injection equipment.2,3 HAV is typically transmitted from person to person through the fecal-oral route but is also suspected to be transmitted through injection drug use or associated behaviors.4
CDC and other organizations recommend multiple strategies to prevent viral hepatitis. Since 1996, the Advisory Committee on Immunization Practices (ACIP) has recommended vaccination with 2 doses of hepatitis A vaccine for persons who use drugs (injection and non-injection) and since 2006 for all children (Table 1) to prevent HAV infection. In addition, ACIP recommends vaccination for persons with chronic liver disease, including disease caused by HCV infection. During conditions of low vaccine supply, CDC recommends 1 dose of hepatitis A vaccine in outbreak settings.15 In Wisconsin, children are not required to receive hepatitis A vaccine before entry into schools or childcare centers. To prevent HBV infection, ACIP has, since 1982, recommended vaccination with 3 doses of hepatitis B vaccine for persons who inject drugs and, since 1991, for all infants. In addition, ACIP recommends vaccination for persons with chronic liver disease, including persons with HCV infection. In Wisconsin, since 1997, state regulations require that children receive 3 doses of hepatitis B vaccine for entry into schools and childcare centers. No vaccine is available to prevent HCV infection. To prevent viral hepatitis and other infections among persons who use drugs, CDC and the US Department of Health and Human Services recommend regular testing for HCV and access to sterile injection and drug preparation equipment through syringe services programs (SSPs).16
Table 1.
Recommendations from the Advisory Committee on Immunization Practices and Wisconsin state regulations about vaccination with hepatitis A vaccine and hepatitis B vaccine and the age in 2018 of persons affected by these recommendations
| Year | Recommender | Type | Persons Recommended for Vaccination | Age of Persons Affected, y |
|---|---|---|---|---|
| Hepatitis A Vaccine | ||||
| 19965 | ACIP | Risk-based | Persons at increased risk, including persons who inject drugs and persons with chronic liver disease | Any age |
| 19965 | ACIP | Risk-based | Children residing in communities with high rates of disease (in Wisconsin, this included the American Indian population) | ≤40 |
| 19996 | ACIP | Risk-based | Children residing in states, counties, and communities with high rates of disease (in Wisconsin, this included children residing in Milwaukee) | ≤37 |
| 20067 | ACIP | Risk-based | Children residing in communities with high rates of disease who had not already been vaccinated (in Wisconsin, this included children residing in Milwaukee) | ≤30 |
| 20067 | ACIP | Universal | All children, beginning at age 1 year | ≤13 |
| Hepatitis B Vaccine | ||||
| 19828 | ACIP | Risk-based | Persons at increased risk, including persons who inject drugs | Any age |
| 19919 | ACIP | Universal | All infants | ≤27 |
| 199510 | ACIP | Universal | All children aged 11-12 years who had not already been vaccinated | ≤35 |
| 1997-2004 phase-in11 | Wisconsin state regulation | Universal | All children entering Wisconsin schools or licensed childcare centers | ≤33 |
| 199912 | ACIP | Universal | All children aged 0-18 years who had not already been vaccinated | ≤37 |
| 200613 | ACIP | Risk-based | Persons with chronic liver disease | Any age |
| 201814 | ACIP | Risk-based | Persons with hepatitis C virus infection | Any age |
Abbreviation: ACIP, Advisory Committee on Immunization Practices.
Despite these national recommendations for the prevention of viral hepatitis, reports of viral hepatitis infections have increased in the United States. Since 2010, fueled by the opioid epidemic, injection drug use and acute HCV infections have increased among young adults nationwide, including in Wisconsin.17 During a similar time frame, acute HBV infections among persons who inject drugs have increased in several US states.18,19 Since early 2017, multiple states have reported outbreaks of HAV infection among persons who use injection or non-injection drugs or are experiencing homelessness.20,21 In Wisconsin, as of 2018, no increases in HAV or HBV infections had been detected among persons who inject drugs (unpublished data for 2017-2018, Wisconsin Division of Public Health [WDPH]).22
National surveys indicate that, among the US general population aged 19-49, 13.4% have received 2 doses of hepatitis A vaccine and 32.9% have received 3 doses of hepatitis B vaccine.23 However, national surveys do not provide state-specific coverage estimates for persons who inject drugs or for smaller adult age ranges (eg, increments of 5 years). This information could be used to assess susceptibility to disease and to guide vaccination efforts. The objective of this study was to evaluate hepatitis A and hepatitis B vaccine coverage among persons who inject drugs in Wisconsin. We focused only on persons who had positive HCV antibody results after testing at an SSP, because these persons are at high risk for infection with HAV and HBV and are systematically reported to WDPH through routine public health surveillance procedures.
Methods
Study Design
We identified persons who inject drugs from HCV surveillance data reported by SSPs that conduct rapid HCV antibody testing in Wisconsin. We focused our assessment on reports from SSPs because SSPs serve a large number of persons who inject drugs, and history of injection drug use is typically complete on reports from SSPs. We then searched the Wisconsin Immunization Registry (WIR),24 Wisconsin’s immunization information system (IIS), to identify the vaccination histories of these persons. The University of Wisconsin–Madison Health Sciences Institutional Review Board certified this activity as a nonresearch activity.
Syringe Services Programs in Wisconsin
WDPH provides funding to 12 fixed-site SSPs in Wisconsin to provide HIV testing, counseling, and referral services. The SSPs provide access to new injection equipment and disposal services, harm-reduction education about safe injection practices and overdose prevention, and naloxone rescue kits. Vaccination services are not currently provided.
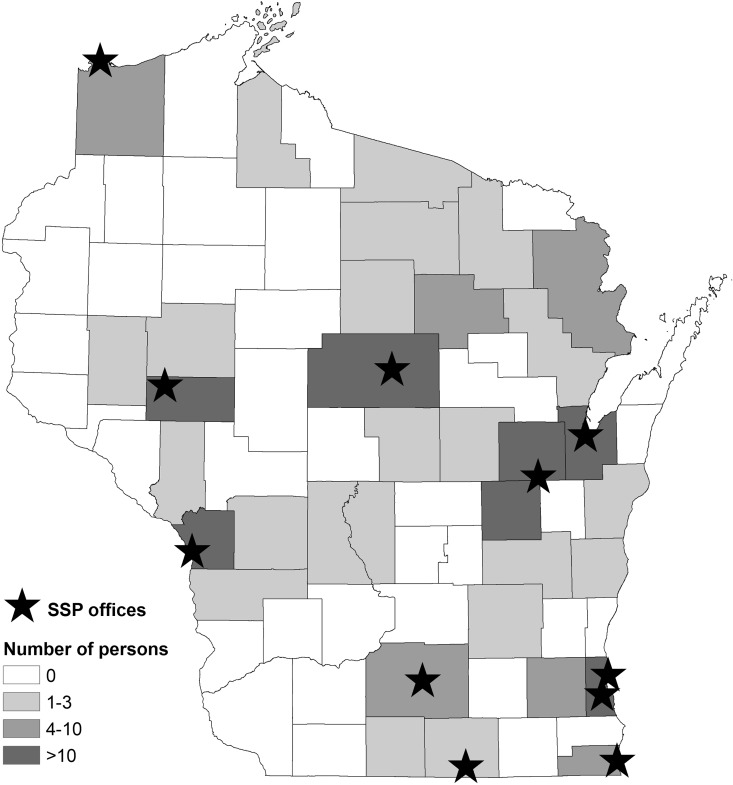
In 2012, the SSPs began offering free rapid HCV antibody testing.25 As of January 1, 2018, 11 SSPs in Wisconsin offered this service (Figure 1). For each person who receives rapid HCV testing, SSP staff members collect information on demographic characteristics and risk factors, including history of injecting drugs. SSP staff members report to WDPH the name, birth date, demographic characteristics, and risk factor data for persons with positive HCV antibody results (indicative of past or present HCV infection).
Figure 1.
Location of 11 syringe services program (SSP) offices that offer rapid hepatitis C virus antibody testing and the number of persons in the study population, by county of residence, Wisconsin, 2018. The study population consisted of persons who inject drugs and had positive hepatitis C antibody test results after testing at an SSP.
Wisconsin Immunization Registry
The WIR is a statewide, population-based IIS that collects and consolidates vaccination histories for Wisconsin residents of all ages. Launched statewide in 2000, the WIR is populated with client demographic information from the WDPH Vital Records Office for all Wisconsin births since 1995. WIR receives new client (for persons born elsewhere or before 1995) and vaccination information from multiple sources, including public and private health care providers, health maintenance organizations, and Wisconsin Medicaid. WDPH estimates that >95% of Wisconsin adults have ≥1 vaccination documented in the WIR.26 The completeness and accuracy of WIR records for adults have not been formally evaluated. However, a previous assessment indicated that WIR records for children were complete and accurate.27 In addition, among pregnant women, the WIR produced higher coverage estimates than Wisconsin’s all-payer claims database.28
Data Collection
HCV infection is a reportable condition in Wisconsin.29 All positive laboratory results, including positive rapid antibody tests, must be reported to WDPH. We defined the study population by using HCV testing data reported by SSPs to WDPH from January 1 through August 31, 2018 (the most recent data available at the time of this assessment). Eligible persons were Wisconsin residents with positive HCV antibody results and a reported history of injecting drugs. On October 12, 2018, we manually searched the WIR for the vaccination records of these persons. We defined a matching client record as having an exact match of first name, last name, and birth date. We included in the study population only persons with WIR client records. For each person in the study population, we extracted data from the WIR on hepatitis A and hepatitis B vaccination dates and the names of the organizations that administered the doses. We included only doses that complied with ACIP-recommended minimum intervals between vaccinations.30,31
Data Analysis
To assess vaccination coverage, we calculated the percentages of persons who received ≥1 dose of hepatitis A vaccine, 2 doses of hepatitis A vaccine (complete hepatitis A vaccine series), and 3 doses of hepatitis B vaccine (complete hepatitis B vaccine series). We assessed receipt of 3 doses of hepatitis B vaccine because at the time of this assessment, a new hepatitis B vaccine32 that requires only 2 doses was in limited use in Wisconsin and had not been received by any of the persons included in our study population. Using narrow age ranges (20-24, 25-29, 30-34, 35-39, 40-49, ≥50 years), we assessed vaccination coverage by the person’s age at the HCV test date. In addition, we assessed vaccination coverage by other characteristics of the study population, including sex (female, male), race/ethnicity (non-Hispanic white, American Indian, Hispanic), location of the SSP where the HCV test was received (northern or western Wisconsin, southeastern Wisconsin), timing of most recent injection drug use (≤6 months ago, >6 months ago), and whether the person ever reported being incarcerated (yes, no).
For comparison, we calculated coverage of hepatitis A vaccine and hepatitis B vaccine among the Wisconsin general population, by age group, using WIR data to determine the number vaccinated, and we used population estimates33 to determine population denominators. For each age group, we calculated 95% confidence intervals (CIs) for the proportion of persons in the study population who were vaccinated. In addition, for each age group, we used 1-sample tests of proportions to assess whether vaccination coverage among the study population differed significantly from vaccination coverage among the general population. We used SAS version 9.434 to conduct all analyses.
Results
From January 1 through August 31, 2018, 11 SSPs reported to WDPH information on 215 persons who had positive HCV antibody test results and a history of injecting drugs. Of these persons, 204 (94.9%) had WIR client records and were included in the study population. The median age was 31 years (range, 20-64 years), 111 (54.4%) were male, and 170 (83.3%) were non-Hispanic white (Table 2). Most reported injecting drugs within the past 6 months (n = 165, 80.9%) and had a history of incarceration (n = 177, 86.8%). One hundred fifty-five (76.0%) persons were reported by 6 SSPs in northern or western Wisconsin. Persons resided in 34 of Wisconsin’s 72 counties (Figure 1).
Table 2.
Number and percentage of persons who received hepatitis A vaccine, hepatitis B vaccine, or both vaccines, by characteristics and number of doses, among the study population,a Wisconsin, 2018b
| Characteristics | Total, No. (%)c | Hepatitis A Vaccine, No. (%)d | 3 Doses of Hepatitis B Vaccine, No. (%)d | Completed Series for Both Vaccines, No. (%)d | |
|---|---|---|---|---|---|
| ≥1 Dose | 2 Doses | ||||
| Overall | 204 (100.0) | 66 (32.4) | 46 (22.5) | 115 (56.4) | 34 (16.7) |
| Age | |||||
| 20-24 | 25 (12.3) | 10 (40.0) | 8 (32.0) | 22 (88.0) | 6 (24.0) |
| 25-29 | 65 (31.9) | 17 (26.2) | 13 (20.0) | 51 (78.5) | 11 (16.9) |
| 30-34 | 44 (21.6) | 14 (31.8) | 7 (15.9) | 24 (54.5) | 4 (9.1) |
| 35-39 | 33 (16.2) | 12 (36.4) | 6 (18.2) | 10 (30.3) | 6 (18.2) |
| 40-49 | 28 (13.7) | 11 (39.3) | 10 (35.7) | 7 (25.0) | 6 (21.4) |
| ≥50 | 9 (4.4) | 2 (22.2) | 2 (22.2) | 1 (11.1) | 1 (11.1) |
| Sex | |||||
| Female | 93 (45.6) | 28 (30.1) | 23 (24.7) | 51 (54.8) | 15 (16.1) |
| Male | 111 (54.4) | 38 (34.2) | 23 (20.7) | 64 (57.7) | 19 (17.1) |
| Race/ethnicitye | |||||
| Non-Hispanic white | 170 (83.3) | 53 (31.2) | 36 (21.2) | 99 (58.2) | 29 (17.1) |
| American Indian | 20 (9.8) | 6 (30.0) | 4 (20.0) | 10 (50.0) | 2 (10.0) |
| Hispanic | 10 (4.9) | 5 (50.0) | 5 (50.0) | 4 (40.0) | 2 (20.0) |
| Location of syringe services program | |||||
| Northern or western Wisconsin | 155 (76.0) | 52 (33.5) | 38 (24.5) | 99 (63.9) | 29 (18.7) |
| Southeastern Wisconsin | 49 (24.0) | 14 (28.6) | 8 (16.3) | 16 (32.7) | 5 (10.2) |
| Most recently injected drugs | |||||
| ≤6 months ago | 165 (80.9) | 52 (31.5) | 39 (23.6) | 94 (57.0) | 29 (17.6) |
| >6 months ago | 39 (19.1) | 14 (35.9) | 7 (17.9) | 21 (53.8) | 5 (12.8) |
| Reported ever being incarceratedf | |||||
| Yes | 177 (86.8) | 57 (32.2) | 38 (21.5) | 103 (58.2) | 30 (16.9) |
| No | 23 (11.3) | 8 (34.8) | 7 (30.4) | 12 (52.2) | 4 (17.4) |
a The study population consisted of persons who inject drugs and had positive hepatitis C antibody test results after testing at a syringe services program.
b Data source: Wisconsin Immunization Registry.24
c Percentages in this column were calculated using the total number of persons in the study population (N = 204) as the denominator.
d Percentages in these columns were calculated using the number in the “Total” column as the denominator.
e Four (2%) persons had unknown race/ethnicity.
f Four (2%) persons did not report information on incarceration.
One hundred forty-seven (72.1%) persons had documentation of vaccination with ≥1 dose of either vaccine. Of these, 56 (38.1%) received a dose during adulthood (aged ≥19), including 20 (35.7%) persons who received ≥1 dose from Wisconsin local health departments and 12 (21.4%) persons who received ≥1 dose from the Wisconsin Department of Corrections.
Sixty-six (32.4%) persons had received ≥1 dose of hepatitis A vaccine, and 46 (22.5%) persons had received 2 doses of hepatitis A vaccine (Table 2). Hepatitis A vaccine coverage varied by age group. The median age at hepatitis A vaccine series completion was 29 (range, 6-45). Ten of 66 (15.2%) persons with ≥1 dose of hepatitis A vaccine were vaccinated on or after the HCV test date.
One hundred fifteen (56.4%) persons had received 3 doses of hepatitis B vaccine (Table 2). The percentage vaccinated with 3 doses of hepatitis B vaccine decreased with increasing age, from 88.0% (22 of 25) of persons aged 20-24 to 30.3% (10 of 33) of persons aged 35-39. The median age at hepatitis B vaccine series completion was 11 years (range, 0-45 years). Of 144 persons with ≥1 dose of hepatitis B vaccine, 7 (4.9%) persons were vaccinated on or after the HCV test date. Thirty-four (16.7%) persons had completed both vaccine series.
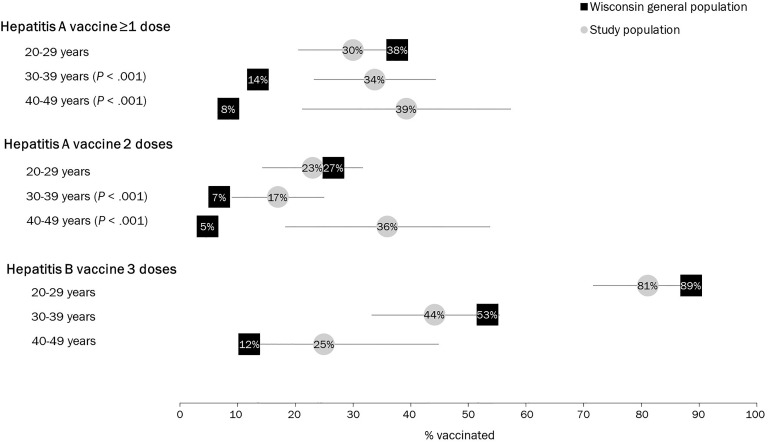
Among persons aged 20-29, 1-dose and 2-dose hepatitis A vaccine coverage estimates among the study population did not differ significantly from the general population. Among persons aged 30-39 and 40-49, 1-dose and 2-dose hepatitis A vaccine coverage estimates were significantly higher among the study population than among the general population. For each age group, hepatitis B vaccine coverage among the study population did not differ significantly from the general population. Hepatitis B vaccine coverage decreased with increasing age among the study population and the general population (Figure 2).
Figure 2.
Hepatitis A and hepatitis B vaccination coverage, by age group, among the study population (ie, persons who inject drugs who had positive hepatitis C antibody test results after testing at a syringe services program) and the general population, Wisconsin, 2018. Gray lines indicate 95% confidence intervals. P values indicate a significant difference between coverage among the study population and coverage among the Wisconsin general population using a 1-sample test of proportions. P < .05 was considered significant. Data source: Wisconsin Immunization Registry.24
Discussion
Despite recommendations for all adults at increased risk of infection to receive hepatitis A and hepatitis B vaccine, among this sample of persons who reported injecting drugs and had positive HCV antibody results (indicative of past or present HCV infection), only 16.7% had completed the vaccine series for both hepatitis A vaccine and hepatitis B vaccine. Poor vaccination coverage among persons who inject drugs is concerning because of their risk for HAV and HBV infection. Most persons in our study population reported injecting drugs in the past 6 months. Furthermore, as indicated by the presence of HCV antibody, it is likely that most persons had previously prepared or injected drugs using unsterile equipment. Poor vaccination coverage is also concerning because, among the estimated 75%-85% of persons with HCV antibody who would be expected to become chronically infected with HCV,35,36 subsequent infection with HAV or HBV might result in further liver damage.
Among our study population, hepatitis A vaccination coverage was low (32.4% had received ≥1 dose and 22.5% had received 2 doses). Low hepatitis A vaccination coverage among adults is expected because universal childhood vaccination was not recommended until 2006. For comparison, 81% of Wisconsin children born after 2006 have received ≥1 dose of hepatitis A vaccine (WDPH, unpublished data, 2018). Among adults aged 30-49, coverage among our study population (a population with risk behavior and chronic liver disease indications for hepatitis A vaccination) was higher than among the general population (a population with no specific indication for hepatitis A vaccination). These findings are similar to national survey results that indicate 2-dose hepatitis A vaccination coverage is 23.7% among adults aged 19-49 with chronic liver conditions and 13.4% among the general population of adults aged 19-49.23
Hepatitis B vaccination coverage was high (88.0%) among adults aged 20-24 in our study population and decreased with increasing age; we observed similar trends among the Wisconsin general population. These findings are consistent with the timing of ACIP recommendations for universal and catch-up vaccination and with Wisconsin law requiring hepatitis B vaccine for school and childcare. Our findings of high hepatitis B vaccination coverage among young adults are consistent with historical results from the National Immunization Survey–Teen, which indicated that coverage among Wisconsin adolescents aged 13-17 in 2008 (persons who were aged 23-27 in 2018) was 87.8%.37 Lower hepatitis B vaccination coverage among older adults is consistent with national survey results that indicate only 46.9% of adults aged 19-49 with chronic liver conditions and 32.9% of adults aged 19-49 in the general population have received 3 doses of hepatitis B vaccine.23 Our findings highlight the importance of evaluating vaccination coverage for more narrow adult age ranges, because aggregate coverage estimates for adults, as provided by national survey results, obscure the important pattern of high coverage among younger adults and low coverage among older adults.
We assessed vaccination coverage among a subset of persons who inject drugs, attended SSPs, and had positive HCV antibody results. More than three-quarters of persons were tested at an SSP in a rural area, where rates of HCV infection among young persons are high and testing is frequent.38 Our study population might have different health care-seeking behaviors or greater access to hepatitis vaccination than persons who inject drugs, choose not to attend SSPs, and do not have diagnosed HCV infection. As a result, vaccination coverage among all persons who inject drugs in Wisconsin might be even lower than among our study population. Overall, our findings suggest that most adults who inject drugs in Wisconsin are susceptible to HAV infection and most adults aged ≥35 are susceptible to HBV infection. These findings are generally consistent with results from a serological assessment of immunity among persons who injected drugs in San Diego during 2009-2010 that indicated more than half of persons aged 18-40 were susceptible to HAV and HBV infection.39
IISs are confidential, population-based databases that collect and consolidate vaccination histories for persons residing within a jurisdiction. IISs can also forecast which vaccinations are currently needed for each person. The National Vaccine Advisory Committee’s Standards for Adult Immunization Practices recommend that all health care providers use IISs to assess each patient’s vaccination status, recommend and administer (or refer to another provider for) the needed vaccinations, and document doses administered in the IIS.40
Expanding the use of IISs and on-site hepatitis vaccination to locations where people who inject drugs receive services could facilitate the identification and vaccination of persons at high risk of infection. Among the persons included in our study who were vaccinated during adulthood, local public health departments and the Wisconsin Department of Corrections were important sources of vaccination. These entities can access the WIR to assess patient vaccination status and document vaccinations administered. Local public health departments in Wisconsin can vaccinate uninsured and underinsured adults, including during follow-up for HCV infection. The Wisconsin Department of Corrections offers hepatitis vaccines to adults entering state correctional facilities. Results of previous studies suggest that offering on-site hepatitis vaccination at SSPs, methadone clinics, jails, and hospital emergency departments can be useful for identifying and vaccinating at-risk populations, including persons who inject drugs.41-47 Incorporating the use of IISs in these settings may further facilitate the identification of high-risk persons in need of vaccinations. Results from an assessment among persons who inject drugs in San Diego indicated that self-report of hepatitis vaccination history among the study population was unreliable and that using an IIS would help identify persons in need of vaccination.39
IISs are also used to assess population vaccination coverage among children, adolescents, adults, and persons in high-risk groups.28,48-51 To our knowledge, our study is the first to use an IIS to evaluate vaccination coverage among persons who inject drugs. This method could be used by other jurisdictions with adult vaccination records in their IISs.26 Alternatively, for jurisdictions that are not able to identify persons who inject drugs or do not have adult vaccination records in their IIS, the information presented in Tables 1 and 2 might provide a general guide to determine which adult age groups have the lowest vaccination coverage.
Limitations
This study had several limitations. First, the persons in our study received HCV testing at SSPs and had positive HCV antibody results; as such, they might not be representative of all persons who inject drugs in Wisconsin. Second, we did not assess immunity to HAV or HBV infection as a result of previous infection. The incidence of HAV and HBV infection in Wisconsin is low,1,22 but anyone in the study population who was immune through previous infection would not be susceptible to disease. Third, vaccination data are provided to the WIR largely voluntarily, and doses administered in other states or before the WIR was implemented might not be documented in the WIR. As a result, vaccination coverage was likely underestimated. Fourth, because of improvements in WIR data completeness over time, coverage might be differentially underestimated by age group, exaggerating lower coverage among older adults. Despite the potential limitations of using an IIS to assess vaccination coverage, in this assessment, most persons who reported injecting drugs had WIR client records, and coverage estimates were consistent with national survey results.
Conclusions
Our findings suggest that most persons who inject drugs in Wisconsin are susceptible to HAV infection and most adults aged ≥35 are susceptible to HBV infection. Incorporating the use of IISs and offering on-site hepatitis vaccination in correctional settings and at locations where persons who inject drugs receive services, such as SSPs, might improve vaccination coverage and prevent future outbreaks. The findings in our study underscore the importance of universal childhood vaccination to ensure protection before becoming at high risk of infection.
Acknowledgments
The authors gratefully acknowledge the AIDS Resource Center of Wisconsin and Sixteenth Street Community Health Center for conducting rapid hepatitis C testing, data collection, and communicable disease reporting; Kimberly Kassander, Wisconsin Division of Public Health, for data entry; and Amy Schumacher, Centers for Disease Control and Prevention (CDC), Wisconsin Division of Public Health, for consultation on data visualization.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partly funded by the National Institute on Drug Abuse, grant no. UG3DA044826. Danielle Sill is funded by the CDC Immunization Information System Sentinel Site Project, grant no. 6 NH23IP000788-05-03. Stephanie Schauer is funded by the CDC Immunization and Vaccines for Children Program, grant no. 6 NH23IP000760-05-02.
ORCID iD: Ruth Koepke, MPH  https://orcid.org/0000-0002-2524-5042
https://orcid.org/0000-0002-2524-5042
Danielle N. Sill, MSPH  https://orcid.org/0000-0001-9785-4069
https://orcid.org/0000-0001-9785-4069
References
- 1. Centers for Disease Control and Prevention. Viral hepatitis surveillance, United States, 2016. https://www.cdc.gov/hepatitis/statistics/2016surveillance/pdfs/2016HepSurveillanceRpt.pdf. Accessed October 31, 2018.
- 2. Bond WW, Favero MS, Petersen NJ, Gravelle CR, Ebert JW, Maynard JE. Survival of hepatitis B virus after drying and storage for one week. Lancet. 1981;1(8219):550–551. doi:19.1016/s0140-6736(81)92877-4 [DOI] [PubMed] [Google Scholar]
- 3. Paintsil E, Binka M, Patel A, Lindenbach BD, Heimer R. Hepatitis C virus maintains infectivity for weeks after drying on inanimate surfaces at room temperature: implications for risks of transmission. J Infect Dis. 2014;209(8):1205–1211. doi:10.1093/infdis/jlt648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Health advisory CDCHAN-00412. 2018. https://emergency.cdc.gov/han/han00412.asp. Accessed July 13, 2018.
- 5. Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45(RR-15):1–30. [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1999;48(RR-12):1–37. [PubMed] [Google Scholar]
- 7. Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-7):1–23. [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Recommendation of the Immunization Practices Advisory Committee (ACIP). Inactivated hepatitis B virus vaccine. MMWR Morb Mortal Wkly Rep. 1982;31(24):317–322, 327–328. [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination: recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep. 1991;40(RR-13):1–25. [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention. Notice to readers update: recommendations to prevent hepatitis B virus transmission—United States. MMWR Morb Mortal Wkly Rep. 1995;44(30):574–575. [PubMed] [Google Scholar]
- 11. Immunization of students. Wis Admin Code DHS 144 (1981).
- 12. Centers for Disease Control and Prevention. Update: recommendations to prevent hepatitis B virus transmission—United States. MMWR Morb Mortal Wkly Rep. 1999;48(2):33–34. [PubMed] [Google Scholar]
- 13. Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part II: immunization of adults [published erratum appears in MMWR Morb Mortal Wkly Rep. 2007;56(42):1114]. MMWR Recomm Rep. 2006;55(RR-16):1–33. [PubMed] [Google Scholar]
- 14. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67(1):1–31. doi:10.15585/mmwr.rr6701a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention. Interim outbreak-specific guidance on hepatitis A vaccine administration. 2017. https://www.cdc.gov/hepatitis/outbreaks/InterimOutbreakGuidance-HAV-VaccineAdmin.htm. Accessed July 13, 2018.
- 16. Belani H, Chorba T, Fletcher F, et al. Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: summary guidance from CDC and the U.S. Department of Health and Human Services. MMWR Recomm Rep. 2012;61(RR-5):1–40. [PubMed] [Google Scholar]
- 17. Zibbell JE, Asher AK, Patel RC, et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health. 2018;108(2):175–181. doi:10.2105/AJPH.2017.304132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris AM, Iqbal K, Schillie S, et al. Increases in acute hepatitis B virus infections—Kentucky, Tennessee, and West Virginia, 2006-2013. MMWR Morb Mortal Wkly Rep. 2016;65(3):47–50. doi:10.15585/mmwr.mm6503a2 [DOI] [PubMed] [Google Scholar]
- 19. Comer M, Matthias J, Nicholson G, Asher A, Holmberg S, Wilson C. Notes from the field: increase in acute hepatitis B infections—Pasco County, Florida, 2011-2016. MMWR Morb Mortal Wkly Rep. 2018;67(7):230–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foster M, Ramachandran S, Myatt K, et al. Hepatitis A virus outbreaks associated with drug use and homelessness—California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(43):1208–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention. Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. Reviewed August 2019 https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed August 15, 2018.
- 22. Bureau of Communicable Diseases, Wisconsin Division of Public Health, Wisconsin Department of Health Services. Wisconsin communicable diseases report 2016. Published 2018 https://www.dhs.wisconsin.gov/publications/p02194.pdf. Accessed October 31, 2018.
- 23. Centers for Disease Control and Prevention. Vaccination coverage among adults in the United States, National Health Interview Survey, 2016. Published 2018 https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2016.html. Accessed July 13, 2018.
- 24. Wisconsin Department of Health Services. Wisconsin Immunization Registry. Revised August 2019 https://www.dhs.wisconsin.gov/immunization/wir.htm. Accessed July 13, 2018.
- 25. Stockman LJ, Guilfoyle SM, Benoit AL, Vergeront JM, Davis JP. Rapid hepatitis C testing among persons at increased risk for infection—Wisconsin, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014;63(14):309–311. [PMC free article] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention. Immunization information systems (IIS): participation rates and maps, 2017. Published 2018 https://www.cdc.gov/vaccines/programs/iis/annual-report-iisar/rates-maps-table.html. Accessed November 9, 2018.
- 27. Koepke R, Petit AB, Ayele RA, et al. Completeness and accuracy of the Wisconsin Immunization Registry: an evaluation coinciding with the beginning of meaningful use. J Public Health Manag Pract. 2015;21(3):273–281. doi:10.1097/PHH.0000000000000216 [DOI] [PubMed] [Google Scholar]
- 28. Koepke R, Schauer SL, Davis JP. Measuring maternal Tdap and influenza vaccination rates: comparison of two population-based methods. Vaccine. 2017;35(18):2298–2302. [DOI] [PubMed] [Google Scholar]
- 29. Communicable Diseases and Other Notifiable Conditions. Wisc Admin Code DHS 110-199, Ch 145 (2018).
- 30. Centers for Disease Control and Prevention. Recommended child and adolescent immunization schedule for ages 18 years or younger. 2019. https://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-combined-schedule-bw.pdf. Accessed July 31, 2018.
- 31. Centers for Disease Control and Prevention. Recommended adult immunization schedule for ages 19 years or older. 2019. https://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule-bw.pdf. Accessed July 31, 2018.
- 32. Schillie S, Harris A, Link-Gelles R, Romero J, Ward J, Nelson N. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67(15):455–458. doi:10.15585/mmwr.mm6715a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wisconsin Department of Health Services. Wisconsin Interactive Statistics on Health: population module. Revised October 2016 https://www.dhs.wisconsin.gov/wish/population/index.htm. Accessed July 31, 2018.
- 34. SAS Version 9.4 [computer software] Cary, NC: SAS Institute, Inc; 2016. [Google Scholar]
- 35. Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132(4):296–305. doi:10.7326/0003-4819-132-4-200002150-00008 [DOI] [PubMed] [Google Scholar]
- 36. Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9(3):393–398. doi:10.1016/j.cid.2005.05.003 [DOI] [PubMed] [Google Scholar]
- 37. Centers for Disease Control and Prevention. TeenVaxView: 2008 through 2017 adolescent hepatitis B (HepB) vaccination coverage trend report. 2018. https://www.cdc.gov/vaccines/imz-managers/coverage/teenvaxview/data-reports/hepb/trend/index.html. Accessed October 31, 2018.
- 38. Wisconsin Department of Health Services. Wisconsin hepatitis C virus surveillance annual review, 2017: newly reported cases, prevalent cases, and trends. 2018. https://www.dhs.wisconsin.gov/publications/p00440-2017.pdf. Accessed October 31, 2018.
- 39. Collier MG, Drobeniuc J, Cuevas-Mota J, Garfein RS, Kamili S, Teshale EH. Hepatitis A and B among young persons who inject drugs—vaccination, past, and present infection. Vaccine. 2015;33(24):2808–2812. doi:10.1016.j.vaccine.2015.04.019 [DOI] [PubMed] [Google Scholar]
- 40. National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory Committee: standards for adult immunization practice. Public Health Rep. 2014;129(2):115–123. doi:10.1177/003335491412900203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bowman S, Grau LE, Singer M, Scott G, Heimer R. Factors associated with hepatitis B vaccine series completion in a randomized trial for injection drug users reached through syringe exchange programs in three US cities. BMC Public Health. 2014;14:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Campbell JV, Garfein RS, Thiede H, et al. Convenience is the key to hepatitis A and B vaccination uptake among young adult injection drug users. Drug Alcohol Depend. 2007;91(suppl 1):S64–S72. doi:10.1016/j.drugalcdep.2006.09.022 [DOI] [PubMed] [Google Scholar]
- 43. Des Jarlais DC, Fisher DG, Newman JC, et al. Providing hepatitis B vaccination to injection drug users: referral to health clinics vs on-site vaccination at a syringe exchange program. Am J Public Health. 2001;91(11):1791–1792. doi:10.2105/ajph.91.11.1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Burr CK, Storm DS, Hoyt MJ, et al. Integrating health and prevention services in syringe access programs: a strategy to address unmet needs in a high-risk population. Public Health Rep. 2014;129(suppl 1):26–32. doi:10.1177/00333549141291S105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Masson CL, Delucchi KL, McKnight C, et al. A randomized trial of a hepatitis care coordination model in methadone maintenance treatment. Am J Public Health. 2013;103(10):e81–e88. doi:10.2105/AJPH.2013.301458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Costumbrado J, Stirland A, Cox G, et al. Implementation of a hepatitis A/B vaccination program using an accelerated schedule among high-risk inmates, Los Angeles County Jail, 2007-2010. Vaccine. 2012;30(48):6878–6882. doi:10.1016/j.vaccine.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 47. Castillo EM, Chan TC, Tolia VM, et al. Effect of a computerized alert on emergency department hepatitis A vaccination in homeless patients during a large regional outbreak. J Emerg Med. 2018;55(6):764–768. doi:10.1016/j.jemermed.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 48. Lopez AS, Cardemil C, Pabst LJ, Cullen KA, Leung J, Bialek SR. Two-dose varicella vaccination coverage among children aged 7 years—six sentinel sites, United States, 2006-2012. MMWR Morb Mortal Wkly Rep. 2014;63(8):174–177. [PMC free article] [PubMed] [Google Scholar]
- 49. Lin X, Rodgers L, Zhu L, Stokley S, Meites E, Markowitz LE. Human papillomavirus vaccination coverage using two-dose or three-dose schedule criteria. Vaccine. 2017;35(43):5759–5761. doi:10.1016/j.vaccine.2017.08.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wagner AL, Shrivastwa N, Potter RC, Lyon-Callo SK, Boulton ML. Pneumococcal and meningococcal vaccination among Michigan children with sickle cell disease. J Pediatr. 2018;196:223–229. [DOI] [PubMed] [Google Scholar]
- 51. Barber A, Muscoplat MH, Fedorowicz A. Coverage with tetanus, diphtheria, and acellular pertussis vaccine and influenza vaccine among pregnant women—Minnesota, March 2013-December 2014. MMWR Morb Mortal Wkly Rep. 2017;66(2):56–59. doi:10.15585/mmwr.mm6602a4 [DOI] [PMC free article] [PubMed] [Google Scholar]