Table 1.
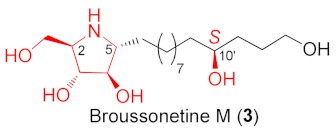
Broussonetine M (3) and analogues synthesized from different cyclic nitrones and alcohols.
| Entry | Cyclic Nitrone | Pyrrolidine | Yield a | Alcohol | Product | Yield b |
|---|---|---|---|---|---|---|
| 1 |
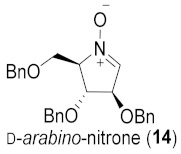
|
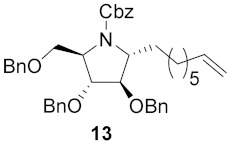
|
64% |
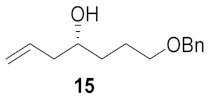
|
|
43% |
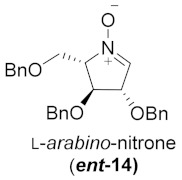
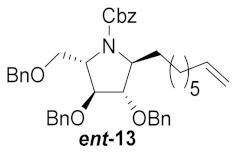
| 2 |
|
|
43% | |||
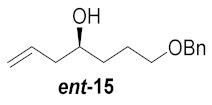
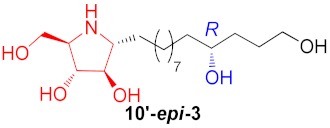
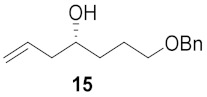
| 3 |
|
|
65% |
|
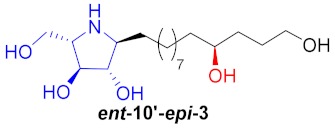
|
41% |
| 4 |
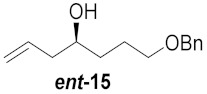
|
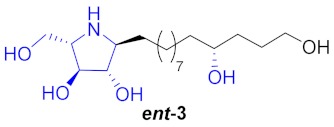
|
45% | |||
| 5 |
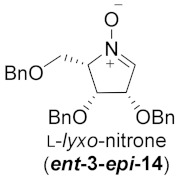
|
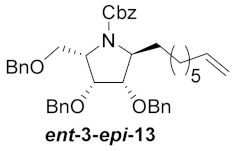
|
64% |
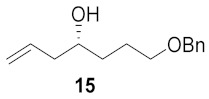
|
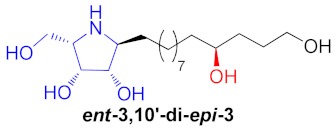
|
40% |
| 6 |
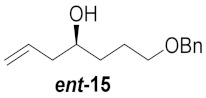
|
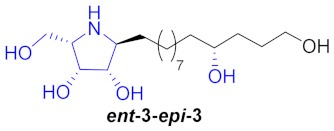
|
41% | |||
| 7 |
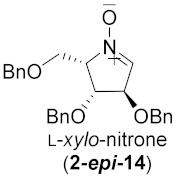
|
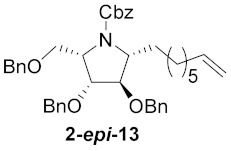
|
71% |
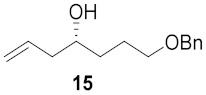
|
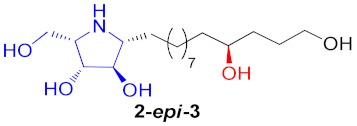
|
43% |
| 8 |
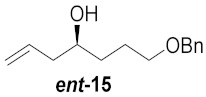
|
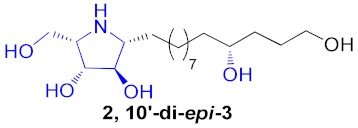
|
43% |
a Total yield in 3 steps starting from cyclic nitrones to the corresponding pyrrolidine. b Total yield in 2 steps starting from pyrrolidine cores to broussonetine M or its analogues.
