Abstract
Four new sesquiterpenoids, named artemivestinolide D–G (1–4) and three known sesquiterpenoids (5–7), were isolated from Artemisia vestita. The structures of these new compounds were determined based on extensive spectroscopic data analyses. Furthermore, the electronic circular dichroism data determined the absolute configurations of the new compounds. The antifeedant and antifungal activities of the isolates were evaluated against third-instar larvae of Plutella xylostella and three plant pathogenic fungi. Compounds 1–7 showed moderate antifeedant activities and compounds 1–4 and 6–7 exhibited antifungal activities.
Keywords: Artemisia vestita, sesquiterpenoids, absolute configuration, antifeedant, antifungal
1. Introduction
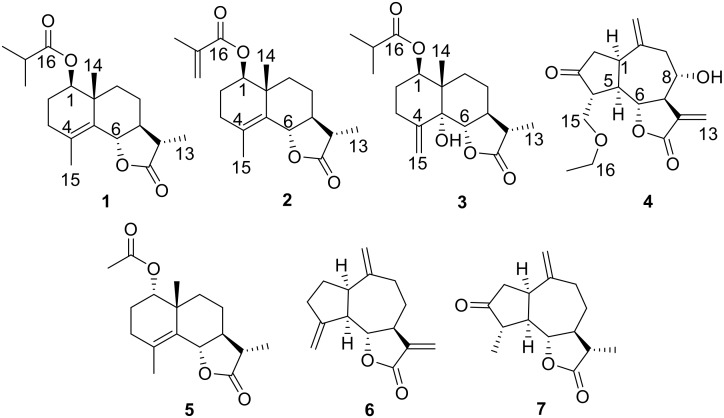
Artemisia (Compositae) species are widespread throughout China and receive much attention due to their remarkable biological activities and structural diversities [1,2,3]. A. vestita is distributed at wasteland and river beaches of China and it has been widely used in traditional Tibetan and Chinese Traditional Medicine for treating various inflammatory diseases [4]. Modern pharmacological studies indicated that ethanol extracts of A. vestita exhibited anti-inflammatory activities [5]. The isolated compounds from A. vestita showed inhibitory effects against NO production induced by LPS [6,7]. Furthermore, A. vestita was found to possess strong insecticidal activity against Sitophilus zeamais and Haemonchus contortus [8,9]. A number of sesquiterpenoids have been isolated from A. vestita and have shown biological activities [4,5,6]. Sesquiterpenoids have important roles in plants’ defense against pests and plant pathogenic fungi so that they can be utilized as natural insecticides and antifungal agents [10,11]. In our continuing research to explore insecticidal and antifungal compounds from plant resources and to extend the knowledge towards the sesquiterpenoids molecules, the whole plants of A. vestita were collected and the extracts were carefully investigated. From the results of our studies on this species, we report herein the isolation, structural determination, and bioactivity of four new sesquiterpenoids, artemivestinolide D–G (1–4), along with three known metabolites, 1α-acetoxyeudesm-4-en-6β,11βH-12,6-olide (5) [6], dehydrocostus lactone (6) [12], and dihydroestafiatone (7) [13] (Figure 1). Herein, we reported the isolation and structural elucidation of the undescribed sesquiterpenoids by extensive spectroscopic techniques (Supplementary Materials) and chemical means. The CD exciton chirality method and calculated ECD spectra were used to determine the absolute configurations of the new compounds. The antifeedant activities against 3rd instar larvae of Plutella xylostella and antifungal activities for plant pathogenic fungi of the isolated compounds were evaluated.
Figure 1.
Structures of sesquiterpenoids 1–7 from Artemisia vestita.
2. Results
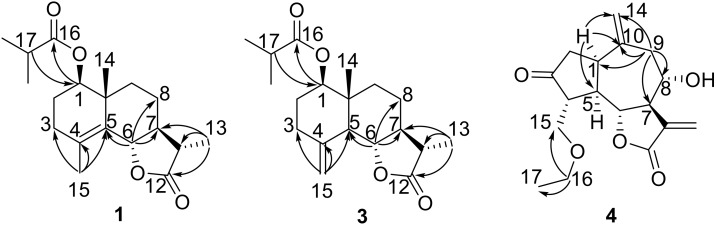
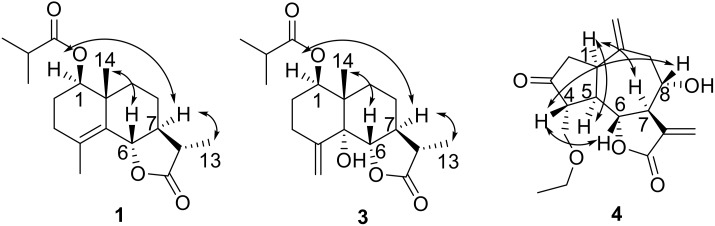
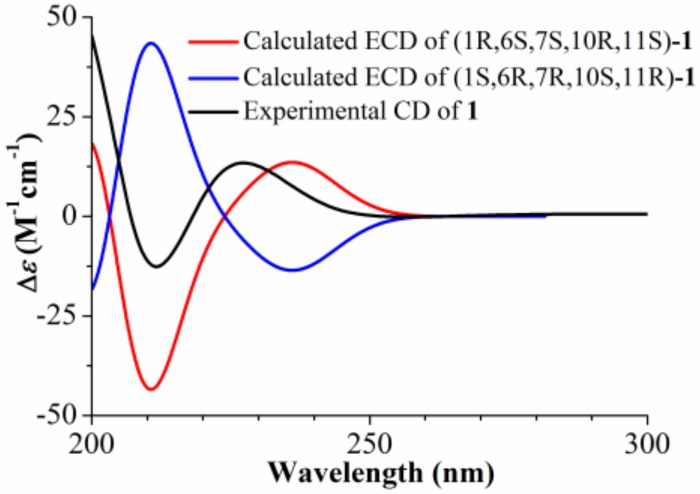
Compound 1 was obtained as a colorless gum and the molecular formula was established as C19H28O4 by HR-ESI-MS at m/z 343.1881 ([M + Na]+; calcd. 343.1880). The IR spectrum of 1 showed the characteristic absorption bands for carbonyl groups (1782 and 1729 cm−1) and double bond (1625 cm−1). The 1H NMR data of 1 (Table 1) exhibited the appearance of one secondary methyl at δH 1.23 (d, J = 7.2 Hz, H3-13), two tertiary methyl groups at δH 1.19 (s, H3-14) and 1.85 (s, H3-15), and two oxymethine protons at δH 4.75 (dd, J = 11.5, 2.8 Hz, H-1) and δH 4.55 (d, J = 11.2 Hz, H-6). Furthermore, methylene proton at δH 2.55 (m, H-17) and two secondary methyl groups at δH 1.17 (d, J = 7.0 Hz, H3-18) and 1.18 (d, J = 7.0 Hz, H3-19) revealed the presence of an isobutyrate group in the molecule. The 13C NMR spectrum (Table 1) indicated 19 carbon resonances, including two ester carbonyls at δC 178.7 (C-12) and 176.6 (C-16), two olefinic carbons at δC 126.4 (C-4) and 128.6 (C-5), and two oxygenated carbons at δC 78.9 (C-1) and 82.6 (C-6). The above NMR spectroscopic data and the degrees of unsaturation suggested that this compound was a sesquiterpene lactone containing three rings. Considering its biological source, it should be an eudesm-12,6-olide derivative [6]. In the HMBC spectrum (Figure 2), correlations were observed from H-1 to C-16 and from H-17 to C-1, indicating that an isobutyrate group was attached to C-1. The HMBC correlations of H3-15 to C-3, C-4, and C-5 allowed the assignment of the olefinic carbons at C-4 and C-5. The HMBC correlations of H-6 to C-5, C-7, and C-8 confirmed the 12,6-olide. The relative stereochemistry of 1 was established by the NOESY spectrum (Figure 3). The NOESY correlations between H3-14 with H-6, and between H-7 with H-1 and H3-13 indicated that H3-14 and H-6 were β-oriented, and H-1, H-7, and H3-13 were α-oriented (Figure 2). The absolute configuration at C-6 of 1 was determined to be S from the CD spectrum using empirical rules of π–π * transition of α,β-unsaturated γ-lactones [6,14], in which a negative Cotton effect was observed at 212 nm. Furthermore, the absolute configuration of 1 was assigned as (1R,6S,7S,10R,11S) based on the fact that the calculated ECD curve for (1R,6S,7S,10R,11S)-1 agreed well with the experimental spectrum for 1 (Figure 4). Thus, compound 1 was elucidated as (1R,6S,7S,10R,11S)-1-isobutyryloxy-eudesma-4(5)-en-12,6-olide, named artemivestinolide D (1).
Table 1.
1H NMR (600 MHz) and 13C NMR (150 MHz) spectroscopic data for compounds 1–4 in CDCl3.
| Position | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | |
| 1 | 4.75, dd (11.5, 2.8) | 78.9 | 4.80, t (7.8) | 79.7 | 5.34, dd (11.0, 4.5) | 74.1 | 3.23, dt (8.8, 4.0) | 40.9 |
| 2a | 1.76, m | 23.8 | 1.82, m | 23.7 | 1.90, m | 26.8 | 2.56, dd (18.2, 8.8) | 44.7 |
| 2b | 1.71, overlapped | 1.80, m | 1.61, m | 2.46, dd (18.2, 4.0) | ||||
| 3a | 2.27, overlapped | 32.8 | 2.27, overlapped | 32.9 | 2.71, m | 29.3 | 216.8 | |
| 3b | 2.01, m | 2.03, m | 2.16, m | |||||
| 4 | 126.4 | 126.5 | 144.6 | 2.38, m | 52.7 | |||
| 5 | 128.6 | 128.5 | 77.1 | 2.90, dd (17.6, 8.3) | 45.9 | |||
| 6 | 4.55, d (11.2) | 82.6 | 4.57, d (11.2) | 82.6 | 4.25, d (9.8) | 81.4 | 4.02, t (8.3) | 81.8 |
| 7 | 1.70, overlapped | 52.8 | 1.68, m | 52.8 | 2.39, overlapped | 45.3 | 3.08, m | 49.7 |
| 8a | 1.90, m | 24.3 | 1.91, m | 24.3 | 1.82, m | 22.7 | 3.94, m | 73.1 |
| 8b | 1.49, m | 1.51, m | 1.47, overlapped | |||||
| 9a | 1.73, overlapped | 38.0 | 1.76, overlapped | 38.1 | 1.72, overlapped | 29.8 | 2.86, dd (12.8, 5.6) | 47.1 |
| 9b | 1.28, m | 1.28, m | 1.46, overlapped | 2.34, dd (12.8, 8.3) | ||||
| 10 | 41.0 | 41.1 | 43.9 | 143.4 | ||||
| 11 | 2.28, overlapped | 41.0 | 2.27, overlapped | 41.1 | 2.31, overlapped | 41.2 | 136.9 | |
| 12 | 178.7 | 178.7 | 179.1 | 169.6 | ||||
| 13a | 1.23, d (7.2) | 12.4 | 1.22, d (7.2) | 12.4 | 1.23, d (7.2) | 12.4 | 6.39, s | 125.2 |
| 13b | 6.31, s | |||||||
| 14a | 1.19, s | 19.8 | 1.20, s | 19.8 | 1.00, s | 14.6 | 5.08, s | 115.9 |
| 14b | 4.87, s | |||||||
| 15a | 1.85, s | 19.8 | 1.82, s | 19.8 | 5.55, s | 112.7 | 3.91, dd (9.1, 2.7) | 68.9 |
| 15b | 5.00, s | 3.70, dd (9.1, 2.7) | ||||||
| 16 | 176.6 | 168.9 | 176.3 | 3.47, m | 66.7 | |||
| 17 | 2.55, m | 34.5 | 136.8 | 2.51, m | 34.4 | 1.16, t (7.0) | 14.9 | |
| 18 | 1.17, d (7.0) | 18.9 | 6.10, s; 5.57, s | 125.3 | 1.16, s | 19.0 | ||
| 19 | 1.18, d (7.0) | 19.1 | 1.96, s | 18.3 | 1.17, s | 19.1 | ||
Figure 2.
Key HMBC correlations of compounds 1, 3, and 4.
Figure 3.
Key NOESY correlations of compounds 1, 3, and 4.
Figure 4.
Calculated and experimental ECD spectra of compound 1.
Compound 2 was obtained as a colorless gum and the molecular formula was established as C19H26O4 by HR-ESI-MS at m/z341.1721 ([M + Na]+; calcd. 341.1723). The NMR data of 2 (Table 1) closely resembled those of 1. The only remarkable difference was a metharcylate group (δH 6.10, 5.57 and 1.96; δC 136.8, 125.3, and 18.3) at C-1 (δC 79.7). This assignment was corroborated by the HMBC correlations of H-1 (δH 4.80, t, J = 7.8 Hz) with C-16 (δC 168.9). The NOESY correlations between H3-14 (δH 1.20, s) with H-6 (δH 4.57, d, J = 11.2 Hz), and between H-7 (δH 1.68, m) with H-1 and H3-13 (δH 1.22, d, J = 7.2 Hz) indicated H3-14 and H-6 were β-oriented, and H-1, H-7, and H3-13 were α-oriented. The absolute configuration at C-6 of 2 was determined as S based on the negative Cotton effect at 211 nm compared with compound 1 in the CD experimental spectrum [6,14]. Thus, compound 2 was elucidated as (1R, 6S, 7S, 10R, 11S)-1-methacryloyloxy-eudesma-4(5)-en-12,6-olide, named artemivestinolide E (2).
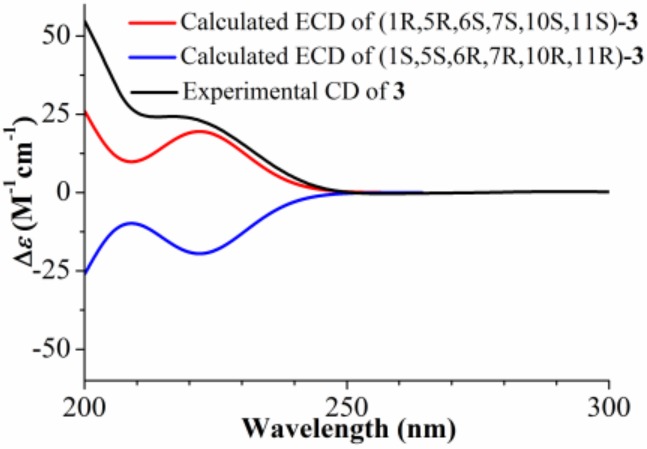
Compound 3 was obtained as a colorless gum and the molecular formula was established as C19H28O5 by HR-ESI-MS at m/z 354.2280 ([M + NH4]+; calcd. 354.2275). The overall appearance of 1H and 13C NMR spectra of 3 are highly similar to those of artemivestinolide C [6]. The most obvious distinction is an isobutyrate group (δH 2.51, 1.16, and 1.17; δC 176.3, 34.4, 19.0, and 19.1) at C-1 (δC 74.1). The cross peaks at H-1 (δH 5.34, dd, J = 11.0, 4.5 Hz) with C-16 in the HMBC spectrum confirmed this (Figure 2). The β-orientation was assigned to C-14 and α-orientation was assigned to 5-OH based on 1H NMR signals of H-1 and H3-14 and 13C NMR signals of C-5, C-10, and C-14 by comparing those with the literature data of eudesmane-type sesquiterpenoid [15]. The NOESY correlations between H3-14 (δH 1.00, s) with H-6 (δH 4.25, d, J = 9.8 Hz), and between H-7 (δH 2.39, m) with H-1 and H3-13 (δH 1.23, d, J = 7.2 Hz) indicated that H3-14 and H-6 were β-oriented, and H-1, H-7, and H3-13 were α-oriented (Figure 3). The absolute configuration at C-5 of 3 was determined as R based on the positive Cotton effect at about 200 nm in the CD experimental spectrum [6]. Furthermore, the absolute configuration of 3 was assigned as (1R,5R,6S,7S,10S,11S) based on the calculated ECD curve for (1R,5R,6S,7S,10S,11S)-3 agreed well with the experimental spectrum for 3 (Figure 5). Thus, compound 3 was elucidated as (1R,5R,6S,7S,10S,11S)-1-isobutyryloxy-eudesma-4(15)-en-12,6-olide, named artemivestinolide F (3).
Figure 5.
Calculated and experimental ECD spectra of compound 3.
Compound 4, obtained as a pale yellow oil, was found to have the molecular formula C17H22O5, as determined through HR-ESI-MS ([M + H]+ peak at m/z 307.1540, calc for C17H23O5, 307.1546). The IR spectrum indicated a hydroxyl group (3431 cm−1) and a carbonyl group (1738 cm−1). The 1H NMR spectrum of 4 (Table 1) showed signals of two oxygenated methane protons at δH 4.02 (t, J = 8.6 Hz, H-6) and 3.94 (m, H-8), two oxygenated methylene protons at δH 3.91 (dd, J = 9.1, 2.7 Hz, H-15a), 3.70 (dd, J = 9.1, 2.7 Hz, H-15b), and 3.47 (m, H2-16), and two terminal double bonds at δH 6.39 (s, H-13a), 6.31 (s, H-13b), 5.08 (s, H-14a), and 4.87 (s, H-14b). The 13C NMR spectrum (Table 1) indicated 17 C-atom signals which were classified by a DEPT experiment into one ketone carbonyl at δC 216.8 (C-3), one unsaturated ester carbonyl at δC 169.6 (C-12), two terminal olefinic carbons at δC 125.2 (C-13) and 115.9 (C-14), and four oxygenated carbons at δC 81.8 (C-6), 73.1 (C-8), 68.9 (C-15), and 66.7 (C-16). The above information suggested that compound 4 was a guaianolide and similar to grosheimin [16] except for a ethoxymethyl group in 4. In the HMBC spectrum (Figure 2), correlations were observed from H2-16 to C-15 and C-17; from H-1 to C-5, C-10, and C-14; and from H2-9 to C-1, C-7, C-8, C-10, and C-14, indicating that the ethoxymethyl group was attached to C-15. The relative configuration of 4 was established by the NOESY experiment, by which the correlations from H-4 to H-6 and H-8, and from H-1 to H-5 and H-7, indicated that H-4, H-6, and H-8 were β-oriented, and H-1, H-5, and H-7 were α-oriented (Figure 3). The absolute configuration of 4 was assigned as (1R,4R,5R,6R,7R,8S) based on the fact that the calculated ECD curve for (1R,4R,5R,6R,7R,8S)-4 agreed well with the experimental spectrum for 4 (Figure 6). The chemical structure of compound 4 was elucidated as (1R,4R,5R,6R,7R,8S)-15-ethoxymethyl-2-oxo-8-hydroxy-guaia-10(14),11(13)-dien-12,6-olide, named artemivestinolide G (4).
Figure 6.
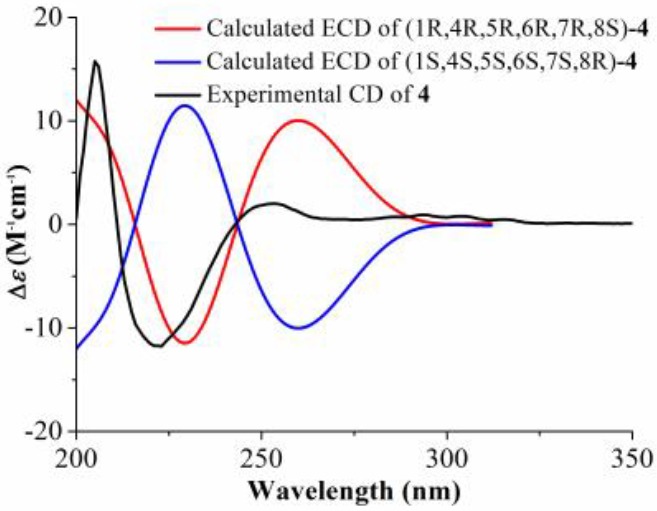
Calculated and experimental ECD spectra of compound 4.
Seven sesquiterpenoids were isolated from A. vestita, including three new eudesmanolides (1–3) and one new guaianolide (4). The result was in agreement with the report suggesting that the sesquiterpenoids were the main secondary metabolites from A. vestita [6,7]. The quality of the sesquiterpenoids we obtained was insufficient and more experiments should be done to optimize the extraction and separation of these compounds.
The isolated sesquiterpenoids were preliminarily investigated for their antifeedant activities against third-instar larvae of P. xylostella and antifungal activities against three pathogenic fungi (Pyricularia oryzae, Botrytis cinerea, and Fusarium oxysporum). As shown in Table 2, all compounds were found to have potential deterrence against the P. xylostella, with EC50 values ranging from 25.3–42.1 μg/cm2. Furthermore, some sesquiterpenoids showed antifungal activities against pathogenic fungi. For P. oryzae, compounds 1 and 6 displayed antifungal activities (MIC of 128 mg/L). For B. cinerea, compounds 1, 2, 4, and 7 showed antifungal effects (MIC of 256 mg/L). For F. oxysporum, compounds 3 and 6 displayed antifungal activities (MIC of 256 mg/L). These results may reveal a way to search for natural product inspired synthetic insecticides and antifungal agents.
Table 2.
Antifeedant activity of compounds 1–7 against P. xylostella.
| Compounds | EC50 (μg/cm2) | Compounds | EC50 (μg/cm2) |
|---|---|---|---|
| 1 | 32.4 | 5 | 33.0 |
| 2 | 37.9 | 6 | 31.9 |
| 3 | 42.1 | 7 | 25.3 |
| 4 | 27.6 | Neem oil | 4.89 |
3. Materials and Methods
3.1. General Experimental Material
Optical rotation data were measured on an Anton Paar MCP 200 Analytical Automatic Polarimeter (Anton Paar GmbH, Graz, Austria). IR data were recorded using a Nicolet Magna-IR 750 spectrophotometer (Thermo Scientific, San Jose, CA, USA). NMR spectra were recorded on AV-600 spectrometer (Bruker, Fällanden, Switzerland). 1H-1H COSY, HSQC, HMBC, and NOESY spectra were measured with the pulse sequence cosygpqf, hsqcetgpsi2, hmbcgpndqf, and noesygpph, respectively (standard sequence, Bruker TOPSPIN 3.1 software) (Bruker, Fällanden, Switzerland). The mixing time (D8) of NOESY spectra was 400 ms. HR-ESI-MS were measured by an Agilent 6520 Q-TOF LC-MS mass spectrometer (Agilent Technologies Co., Ltd., Palo Alto, CA, USA). Analytical and preparative TLC were run on silica gel plates (GF254, Qingdao Haiyang Chemical Co., Ltd., Qingdao, China). Spots were visualized by spraying with 10% H2SO4, followed by heating. Column chromatography (CC) was performed on silica gel (200-300 mesh, Qingdao Haiyang Chemical Co., Ltd., Qingdao, China) and Sephadex LH-20 (Pharmacia Fine Chemical Co., Ltd., Uppsala, Sweden). Acarbose was purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Plant Material
The A. vestita was collected in Alxa left Banner, Inner Mongolia Autonomous Region, People’s Republic of China, in August 2017, and identified by one of the authors (H.-B.W.). A voucher specimen (NO. 20170839) was deposited in the herbarium of the College of Life and Environmental Sciences, Minzu University of China, Beijing, People’s Republic of China.
3.3. Extraction and Isolation
The chopped and dried A. vestita (5.0 kg) were pulverized and extracted three times with MeOH (each for seven days) at room temperature. After filtration, the filtrate was concentrated under reduced pressure to yield a residue (225.0 g ) and then was suspended in water and partitioned successively with petroleum ether, CHCl3, EtOAc, and n-butanol, to afford five fractions. The CHCl3 fraction (45.5 g) was subjected to silica gel CC (1.0 kg) eluting with PE−CHCl3 (15:1−0:1, gradient system). On the basis of TLC analysis, five fractions A−E were obtained. Fraction A (4.2 g) was eluted with PE−EtOAc (12:1) on CC, further purified by Sephadex LH-20 (CHCl3−MeOH, 1:1) to afford 1 (6.1 mg), 2 (4.1 mg), and 5 (3.9 mg). Fraction B (1.6 g) was subjected to CC eluting with PE−EtOAc (8:1) to afford 3 (3.3 mg), and 6 (1.9 mg). Fraction C (1.9 g) was subjected to CC eluting with PE−CHCl3 (3:1) to afford 4 (1.6 mg) and 7 (2.2 mg) [17].
3.4. Spectroscopic Data
Artemivestinolide D (1): Colorless gum; +24.5 (c = 0.12, MeOH); UV (CH3CN) λmax (log ε) 200 (2.42) nm; ECD (CH3CN) λmax (Δε) 212 (−5.1), 227 (+5.37) nm; IR (KBr) νmax 1782, 1729, 1625, 1157 cm−1; 1H and 13C NMR data (see Table 1); HR−ESI−MS m/z 343.1881 (calcd for C19H28NaO4, 343.1880).
Artemivestinolide E (2): Colorless gum; +27.6 (c = 0.16, MeOH); UV (CH3CN) λmax (log ε) 231 (3.39) nm; ECD (CH3CN) λmax (Δε) 211 (−34.4), 233 (+2.94) nm; IR (KBr) νmax 1781, 1716, 1634, 1166 cm−1; 1H and 13C NMR data (see Table 1); HR−ESI−MS m/z 341.1721 (calcd for C19H26NaO4, 341.1723).
Artemivestinolide F (3): Colorless gum; +17.3 (c = 0.13, MeOH); UV (CH3CN) λmax (log ε) 200 (1.22) nm; ECD (CH3CN) λmax (Δε) 200 (+45.7) nm; IR (KBr) νmax 1773, 1726, 1613, 1157 cm−1; 1H and 13C NMR data (see Table 1); HR−ESI−MS m/z 354.2280 (calcd for C19H32NO5, 354.2275).
Artemivestinolide G (4): Pale yellow oil; +6.7 (c = 0.07, MeOH); UV (CH3CN) λmax (log ε) 210 (0.35) nm; ECD (CH3CN) λmax (Δε) 205 (+27.6), 223 (−20.6), 253 (+3.5) nm; IR (KBr) νmax 3431, 1738, 1634, 1385, 1120 cm−1; 1H and 13C NMR data (see Table 1); HR−ESI−MS m/z 307.1540 (calcd for C17H23O5, 307.1546).
3.5. Antifeedant Bioassay
The antifeeding effect was estimated through a no-choice assay using leaf disc method [11]. Leaf discs (1 cm × 1 cm) of cabbage were cut and immersed in acetone solution with compounds for 5 s, and air dried at room temperature. Five treated leaf discs and 10 larvae were placed together in one 90 mm diameter Petri dish. Residual leaf area of the control and the treatment were measured separately by graph paper at 24 h post treatment and the percentage antifeedant index was then determined using the following equation: antifeedant index (%) = [(CK−T)/CK] × 100, where CK refers to the leaf area consumed in the control disc while T indicates the leaf area consumed in the treatment.
3.6. Antifungal Bioassay
The test phytopathogenic fungi used in this study were P. oryzae, B. cinerea, and F. oxysporum. The microdilution method, with 96-well microliter plates using a potato dextrose (PD) medium, was used to evaluate antifungal activity of the compounds [18]. After incubation for 48 h at 28 ± 0.5 °C, minimum inhibitory concentration (MIC) was taken as the lowest concentration of the test compounds in 96-well plate, in which no microbial growth could be observed.
4. Conclusions
In this study, seven sesquiterpenoids, including four new compounds, were isolated from A. vestita, and their structures were primarily elucidated on the basis of NMR and MS studies. The absolute configuration of the new compounds was determined by CD exciton chirality and calculated ECD methods. The isolated compounds were tested for antifeedant activities against third-instar larvae of P. xylostella and antifungal activities against three pathogenic fungi, with all compounds showing moderate antifeedant activities and some compounds exhibiting antifungal activities.
Supplementary Materials
Supplementary material relating to this article can be accessed online.
Author Contributions
Conceptualization, S.S.; Data curation, Y.-H.D., H.-T.W., Y.M. and H.-B.W.; Funding acquisition, S.S.; Methodology, Y.-H.D., H.-T.W. and H.-B.W.; Project administration, S.S.; Writing—original draft, Y.-H.D., H.-T.W., S.S., Y.M. and J.C.F.; Writing—review & editing, Y.-H.D., H.-T.W. and S.S.
Funding
This work was supported by the National Science Foundation of China [No. 31570407]; Building World-Class University (Discipline) Funds [No. 10301-0190040129].
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Bora K.S., Sharma A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011;49:101–109. doi: 10.3109/13880209.2010.497815. [DOI] [PubMed] [Google Scholar]
- 2.Tan R.X., Zheng W.F., Tang H.Q. Biologically active substances from the genus Artemisia. Planta Med. 1998;64:295–302. doi: 10.1055/s-2006-957438. [DOI] [PubMed] [Google Scholar]
- 3.Abad M.J., Bedoya L.M., Apaza L., Bermejo P. The Artemisia L. genus: A review of bioactive essential oils. Molecules. 2012;17:2542–2566. doi: 10.3390/molecules17032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin Y., Gong F.Y., Wu X.X., Sun Y., Li Y.H., Chen T., Xu Q. Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita. J. Ethnopharmacol. 2008;120:1–6. doi: 10.1016/j.jep.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 5.Sun Y., Li Y.H., Wu X.X., Zheng W., Guo Z.H., Li Y., Chen T., Hua Z.C., Xu Q. Ethanol extract from Artemisia vestita, a traditional Tibetan medicine, exerts anti-sepsis action through down-regulating the MAPK and NF-κB pathways. Int. J. Mol. Med. 2006;17:957–962. doi: 10.3892/ijmm.17.5.957. [DOI] [PubMed] [Google Scholar]
- 6.Tian S.H., Chai X.Y., Zan K., Zeng K.W., Tu P.F. Three new eudesmane sesquiterpenes from Artemisia vestita. Chin. Chem. Lett. 2013;24:797–800. doi: 10.1016/j.cclet.2013.05.020. [DOI] [Google Scholar]
- 7.Tian S.H., Chai X.Y., Zan K., Zeng K.W., Guo X., Jiang Y., Tu P.F. Arvestolides A–C, new rare sesquiterpenes from the aerial parts of Artemisia vestita. Tetrahedron Lett. 2013;54:5035–5038. doi: 10.1016/j.tetlet.2013.07.023. [DOI] [Google Scholar]
- 8.Chu S.S., Liu Q.R., Liu Z.L. Insecticidal activity and chemical composition of the essential oil of Artemisia vestita from China against Sitophilus zeamais. Biochem. Syst. Ecol. 2010;38:489–492. doi: 10.1016/j.bse.2010.04.011. [DOI] [Google Scholar]
- 9.Irum S., Ahmed H., Mukhtar M., Mushtaq M., Mirza B., Donskow-Łysoniewska K., Qayyum M., Simsek S. Anthelmintic activity of Artemisia vestita Wall ex DC. and Artemisia maritima L. against Haemonchus contortus from sheep. Vet. Parasitol. 2015;212:451–455. doi: 10.1016/j.vetpar.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 10.Mann R.S., Kaufman P.E. Natural product pesticides: Their development, delivery and use against insect vectors. Mini Rev. Org. Chem. 2012;9:185–202. doi: 10.2174/157019312800604733. [DOI] [Google Scholar]
- 11.Wu H.B., Wu H.B., Wang W.S., Liu T.T., Qi M.G., Feng J.C., Li X.Y., Liu Y. Insecticidal activity of sesquiterpene lactones and monoterpenoid from the fruits of Carpesium abrotanoides. Ind. Crops Prod. 2016;92:77–83. doi: 10.1016/j.indcrop.2016.07.046. [DOI] [Google Scholar]
- 12.Neves M., Morais R., Gafner S., Stoeckli-Evans H., Hostettmann K. New sesquiterpene lactones from the Portuguese liverwort Targionia lorbeeriana. Phytochemistry. 1999;50:967–972. doi: 10.1016/S0031-9422(98)00648-7. [DOI] [Google Scholar]
- 13.Wu X.L., Xiong X.J., Lu W.Q., Huang H., Shen Y.H., Wu Z.J., Chen W.S. New sesquiterpenenoids from Ainsliaea yunnanensis. Molecules. 2016;21:1031. doi: 10.3390/molecules21081031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida I., Kuriyama K. The π-π circular dichroism of α,β-unsaturated γ-lactones. Tetrahedron Lett. 1974;15:3761–3764. doi: 10.1016/S0040-4039(01)92002-7. [DOI] [Google Scholar]
- 15.Miyase T., Fukushima S. Studies on sesquiterpene glycosides from Sonchus oleraceus L. Chem. Pharm. Bull. 1987;35:2869–2874. doi: 10.1248/cpb.35.2869. [DOI] [Google Scholar]
- 16.Barbetti P., Fardella G., Chiappini I., Scarcia V., Candiani A.F. New cytotoxic selenoderivatives of guaianolides. Eur. J. Med. Chem. 1989;24:299–305. doi: 10.1016/0223-5234(89)90013-5. [DOI] [Google Scholar]
- 17.Bucar F., Wube A., Schmid M. Natural product isolation—How to get from biological material to pure compounds. Nat. Prod. Rep. 2013;30:525–545. doi: 10.1039/c3np20106f. [DOI] [PubMed] [Google Scholar]
- 18.Wu H.B., Liu T.T., Lian Y.X., Wang W.S. Two new eremophilenolides from the roots of Ligulariopsis shichuana and their anti-phytopathogenic fungal and antifeedant activities. Nat. Prod. Res. 2019;33:1442–1448. doi: 10.1080/14786419.2017.1419240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.