Abstract
Rationale:
Androgen deprivation therapy (ADT) is an effective treatment for prostate cancer, but induces profound cognitive impairment. Little research has addressed mechanisms underlying these deficits or potential treatments. This is an unmet need to improve quality of life for prostate cancer survivors.
Objectives:
We investigated mechanisms of cognitive impairment after ADT in rats and potential utility of the multi-modal serotonin-targeting drug, vortioxetine, to improve the impairment, as vortioxetine has specific efficacy against cognitive impairment in depression.
Methods:
Male Sprague-Dawley rats were surgically castrated. Vortioxetine (28 mg/kg/day) was administered in the diet. The Attentional Set-Shifting Test was used to assess medial prefrontal cortical (mPFC) executive function. Afferent-evoked field potentials were recorded in mPFC of anesthetized rats after stimulating the ventral hippocampus (vHipp) or medial dorsal thalamus (MDT). Gene expression changes were assessed by microarray. Effects of vortioxetine on growth of prostate cancer cells were assessed in vitro.
Results:
ADT impaired cognitive set-shifting and attenuated responses evoked in the mPFC by the vHipp afferent, but not the MDT. Both the cognitive impairment and attenuated vHipp-evoked responses were reversed by chronic vortioxetine treatment. Preliminary investigation of gene expression in the mPFC indicates that factors involved in neuronal plasticity and synaptic transmission were down-regulated by castration and up-regulated by vortioxetine in castrated animals. Vortioxetine neither altered the growth of prostate cancer cells in vitro, nor interfered with the anti-proliferative effects of the androgen antagonist, enzalutamide.
Conclusions:
These results suggest that vortioxetine may be useful in mitigating cognitive impairment associated with ADT for prostate cancer.
Keywords: cognitive flexibility, antidepressant, androgen, medial prefrontal cortex, ventral hippocampus, medial dorsal thalamus, prostate cancer
Introduction:
Androgen deprivation therapy (ADT) is a mainstay treatment for androgen-dependent late stage prostate cancer. This type of cancer is relatively slow growing and has a 5-year survival rate of 99% (Siegel et al. 2018). Between 80–90% of prostate cancer cases are androgen-sensitive, and approximately 45% of all patients will undergo ADT at some point during their treatment regimen (Denis and Griffiths 2000; Gilbert et al. 2011). However, ADT is accompanied by serious side effects, including profound cognitive impairment in approximately 47–69% of patients who undergo treatment (Nelson et al. 2008). These impairments are primarily seen in cognitive domains associated with spatial cognition, executive function, attention, and memory (Green et al. 2002; Nelson et al. 2008). These effects present within 6–12 months after beginning treatment, and increase in severity with duration of treatment (Gonzalez et al. 2015). Along with potential cognitive decline after ADT, clinical studies have also shown that men who undergo ADT have increased risk of subsequent diagnoses of Alzheimer’s disease and dementia (Nead et al. 2016; Nead et al. 2017). Not only does this diminish the quality of life for patients themselves, it can also negatively impact their families and caregivers. There are currently no existing treatments for cognitive impairment associated with ADT, and the risk of cognitive decline could deter patients from selecting ADT. Finding interventions that can mitigate these detrimental effects may increase the number of patients who receive ADT for prostate cancer, and improve the long-term quality of life for those patients.
The medial prefrontal cortex (mPFC) has been implicated as one of the regions associated with ADT-induced cognitive decline. The mPFC is important for emotional regulation, working memory and executive function, including cognitive flexibility. fMRI studies of ADT patients indicate hypoactivity and reduced functional connectivity of the mPFC at rest (Chao et al. 2012). Follow up studies found these patients also had reduced gray matter volume in the mPFC and impaired performance in working memory tasks (Chao et al. 2013). Other studies have reported significant impairment of visuospatial memory after ADT, which implicates dysfunction of the hippocampus (Hipp) (Jamadar et al. 2012; Jenkins et al. 2005). Thus, a brain circuit involving the Hipp and the mPFC may be compromised by ADT, but there is little research into the neurobiological mechanisms underlying these impairments. Even less is known about possible interventions that may alleviate the detrimental cognitive effects of ADT.
Vortioxetine (Trintellix) is an FDA-approved multimodal antidepressant that uniquely improves cognitive impairment associated with major depressive disorder. Similar to serotonin-selective reuptake inhibitors, vortioxetine blocks the serotonin transporter, and effectively increases serotonergic neurotransmission in the mPFC and Hipp (Sanchez et al. 2015). It also selectively interacts with several other pre- and post-synaptic serotonin receptors, including acting as a 5-HT3, 5-HT1D and 5-HT7 receptor antagonist, a 5-HT1B partial agonist, and a 5-HT1A agonist (Bang-Anderson et al. 2011). These additional receptor interactions may contribute to vortioxetine’s ability to improve cognitive impairment (Wallace et al. 2014), and previous results from our lab have shown that chronic dietary administration of vortioxetine effectively rescued deficits in reversal learning, a cognitive process mediated by the orbitofrontal cortex, induced by chronic intermittent cold-stress (Wallace et al. 2014).
Based on these findings, we hypothesized that vortioxetine may improve cognitive processes mediated in the mPFC that are impaired after androgen deprivation. Cognitive flexibility is an executive function that requires the mPFC, and we can assess mPFC-dependent cognitive flexibility in rats by measuring performance on the extra dimensional (ED) set-shifting task of the Attentional Set-shifting Test (AST) (Bondi et al. 2010; Bondi et al. 2008; Fucich et al. 2016; Jett and Morilak 2013). Therefore, the purpose of the present experiments was to investigate mechanisms underlying impairments in set-shifting induced by physical castration as a rodent model of ADT, and to test the hypothesis that vortioxetine can reverse these deficits. Portions of this work have been presented in abstract form (Sharp et al. 2017).
Methods:
Animals
Adult male castrated and uncastrated control Sprague-Dawley rats were obtained from Envigo (USA). Castrations were performed by the vendor approximately 3–5 days prior to shipment. Animals were approximately 60 days old and weighed 225–250 g upon arrival. Beginning 8–10 days after castration, rats were singly housed for 17 days prior to testing. Housing conditions included a 12/12 hr light cycle (lights on at 07:00 hrs) and free access to food and water, except for the period of food restriction prior to the AST procedure. Behavioral testing, electrophysiological recording, and tissue collection all occurred during the light phase, starting at approximately 09:00 and completed by 17:00 hrs. All procedures were approved by the University of Texas Health Science Center at San Antonio Institutional Animal Care and Use Committee and complied with National Institute of Health guidelines.
Vortioxetine treatment.
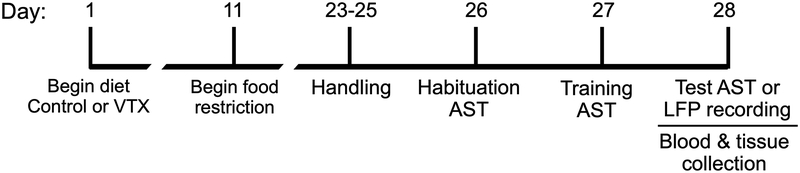
Vortioxetine was generously provided by H. Lundbeck A/S, and the chow was prepared by Research Diets, Inc. Standard rat chow (Purina #5001) was used as control diet and served as the base for drug chow. Vortioxetine diet contained 0.6 g of drug per kg chow, corresponding to a dose of 28 mg/kg/day during the 7-day period of food restriction prior to testing. At this dose, plasma levels of drug are within the range of therapeutic efficacy, and produce 60–95% occupancy of relevant drug targets in the brain (Wallace et al. 2014). Rats were placed on either control or vortioxetine diet beginning approximately 8–10 days after castration, corresponding with the first day of single housing. They were on diet for a total of 17 days, free feeding for the first 10 days then restricted to 14 grams of food/day for 7 days through testing (Fig. 1).
Fig. 1.
Timeline of experiments. Different cohorts of rats were used for each experiment.
Attentional Set-Shifting Test (AST).
The AST was conducted as previously described (Bondi et al. 2008). The test apparatus (75 × 44 × 30 cm) was a white wooden box with a removable start gate forming a holding area at the front. The distal section of the box was divided into two sections by a Plexiglas panel. A small terracotta pot (diameter 7 cm, depth 6 cm) was placed in each of the two sections. The pots were differentiated by two sensory cues (Table 1), the textured medium that filled each pot and an odor applied to the rim. The reward consisted of a ¼ piece of Honey Nut Cheerio (General Mills Cereals, Minneapolis, MN, USA) buried 2 cm below the surface of the positive pot. Cheerio powder was lightly sprinkled over the medium of both pots to mask the smell of the reward. For 3 days prior to habituation (day 23–25), two terracotta pots were placed in the home cage and rats were handled for 5 min per day. The AST procedure began on day 26 and took 3 days.
Table 1.
Attentional Set-shifting Test (AST) Procedure. The example shown is based on odor as the initial positive discriminatory cue and medium as the distractor. In the extra-dimensional set-shift, the medium becomes informative, signaling the location of the reward and the odor becomes the distractor. This phase of the AST is mediated by function of the mPFC.
| Discrimination Stages in the Attentional Set-Shifting Test | ||||
|---|---|---|---|---|
| Discrimination Stage | Dimensions | Example Combinations | ||
| Relevant | Irrelevant | (+) | (−) | |
| Simple (SD) | Odor | Clove/Sawdust | Nutmeg/Sawdust | |
| Compound (CD) | Odor | Medium | Clove/Raffia | Nutmeg/Metal Confetti |
| Clove/Metal Confetti | Nutmeg/Raffia | |||
| Reversal (R1) | Odor | Medium | Nutmeg/Raffia | Clove/Metal Confetti |
| Nutmeg/Metal Confetti | Clove/Raffia | |||
| Intra Dimensional Shift (ID) | Odor | Medium | Rosemary/Wood Balls | Cinnamon/Plastic Beads |
| Rosemary/Plastic Beads | Cinnamon/Wood Balls | |||
| Reversal (R2) | Odor | Medium | Cinnamon/Plastic Beads | Rosemary/Wood Balls |
| Cinnamon/W ood Balls | Rosemary/Plastic Beads | |||
| Extra Dimensional Shift (ED) | Medium | Odor | Velvet/Citronella | Crepe/Thyme |
| Velvet/Thyme | Crepe/Citronella | |||
In half the rats, odor will be relevant first, as shown, and in half the rats, medium will be first.
Habituation:
On day 26, rats were trained to dig for the reward in unscented pots filled with increasing amounts of sawdust, first in their home cage, then in the testing arena. Completion of habituation was indicated by 3 successful retrievals of the Cheerio from both of the sawdust filled pots in the arena.
Training:
On day 27, rats were trained in the testing arena to make a simple discrimination (SD) using each stimulus dimension alone, first discriminating by odor (lemon or rosewood) with sawdust-filled pots, and then discriminating by digging medium (felt or paper strips) in unscented pots. One pot was placed in each of the divided sections of the arena, but only one pot was baited with the reward. To begin, the rat was placed in the start box, and a trial began by raising the gate. The rat was then allowed to explore freely until it made a choice by digging in one of the pots. If the rat dug in the incorrect pot first, it was returned to the start gate, the trial was terminated, and an error was scored. If the rat chose the correct pot, it was allowed to consume the reward, then returned to the start box, and a correct choice was scored. Criterion for successful mastery of a task was 6 consecutive correct choices in each stimulus dimension.
Testing:
On day 28, testing consisted of a series of discriminations similar to the training procedure, in which the rats learned to locate the food reward based on association with a cue in one of the two stimulus dimensions. For each task, the discriminative dimension, and the positive cue within that dimension, were varied as outlined in Table 1. Animals were counter-balanced so that half started with odor as the initial discriminating dimension, and half started with medium (the example in Table 1 starts with odor as the first relevant dimension). Once they mastered a given contingency, indicated by reaching the criterion of 6 consecutive correct trials, the rules were changed and the rat needed to adapt to learn the new rule. In the first 5 stages, which include a simple discrimination, complex discrimination, reversal learning, new acquisition and second reversal, the rats formed a higher-order learning strategy known as a “cognitive set,” in which they learned that only one stimulus dimension is relevant, regardless of how the rules change. The final stage of the test, the extra-dimensional (ED) set-shift, required the rats to abandon their cognitive set to learn that the previously irrelevant dimension has now become relevant, signaling the location of the reward, and the previously relevant cue now becomes the distractor. This type of cognitive flexibility is dependent on the function of the mPFC. For each phase of the test, the dependent measure is the number of trials required for the rat to reach the criterion of 6 consecutive correct trials on the ED set-shifting task.
Evoked Local Field Potentials to Assess mPFC Response to Afferent Input.
In a separate cohort of rats, afferent-evoked local field potentials were recorded in the mPFC as previously described (Jett et al. 2017). Recordings occurred on day 28 post-surgery, corresponding with the test day for AST. Rats were anesthetized with chloral hydrate (400 mg/kg, i.p., supplemented 10% as needed through the duration of recording), and placed in a stereotaxic apparatus on a metal plate heating pad to maintain body temperature at 37°C. An insulated stainless steel bipolar concentric stimulating electrode was positioned in the medial dorsal thalamus (MDT) (coordinates from bregma: AP −2.6, ML +0.8, DV −5.4mm) or in the ventral hippocampus (vHipp) (AP: −6.0, ML: +5.4, DV: −7.5mm) and a tungsten recording electrode was placed in the ipsilateral mPFC (AP +3.0, ML +0.6, DV −3.5mm). Signal was filtered (low cutoff 0.3 Hz, high cutoff 1000 Hz, sampling 2000 Hz) and digitized using PowerLab (ADInstruments). The response of the mPFC was recorded after an equilibration period of 15 minutes. The amplitude of the mPFC response evoked by stimulating the MDT or vHipp (0.1 Hz, 260 μsec pulse width, 100–600 μA in 100 μA steps, 30 pulses at each stimulation intensity) was used to generate a current response curve. For MDT-evoked responses, the magnitude of response was measured from the peak of the first negative deflection, occurring at approximately 5–8 msec after stimulation, to the peak of the positive deflection that immediately followed at 12–15 msec. For vHipp-evoked responses, the magnitude of response was measured from the peak of the first negative deflection, occurring at approximately 20–25 msec after stimulation, to the peak of the positive deflection at 30–35 msec that immediately followed. Electrode placement was confirmed histologically. Cases in which the electrodes were located outside the target regions were excluded from the study. This resulted in the exclusion of 5 rats (2 from MDT and 3 from vHipp recordings). Numbers reported in the results below refer only to animals included in the final analyses.
Whole genome microarray analysis of gene expression.
In a separate cohort, rats were sacrificed on day 28, corresponding to the testing day for the AST or electrophysiological recording. The mPFC was bluntly dissected and immediately flash frozen in 2-methylbutane on dry ice, then stored at −80°C. Right and left hemispheres were represented equally across all four groups. RNA was isolated using Qiazol lysis buffer (Qiagen) and the Direct-zol mini prep RNA isolation kit (Zymo Research). RNA quality was determined with an Agilent Bioanalyzer. Only samples with RNA Integrity Number (RIN) >7 were used. Cyanine 3-labeled cRNA probes were created from 100–200 ng RNA with the use of the Low Input Quick Amp Labeling kit (Agilent) according to the manufacturer’s directions. The cRNAs were then hybridized to the Agilent G3 Rat GE 8×60K v2 Microarray Kit at 65°C for 17 hrs. The arrays were washed and the fluorescent signals were detected using an Agilent SureScan microarray scanner. Feature extraction was performed on the raw data. The feature-extracted data was imported into GeneSpring GX software (Agilent) for statistical analysis of gene expression to determine genes and pathways that were differentially expressed in the groups.
Expression data were visualized using hierarchical clustering dendrograms and heatmaps. Transcript expression intensities were normalized and analyzed with the LIMMA R package that implements the false discovery rate adjustment to account for multiple transcript tests within comparisons. Differential expression due to castration and vortioxetine treatment were estimated using linear model contrasts applied to log-intensity measures, including interactions. Differential expression due to vortioxetine alone was estimated using only non-castrated intact samples. Differential expression due to castration alone was estimated using only control diet (non-vortioxetine) samples. Main effects and interactions between vortioxetine and castration were estimated using all samples with ANOVA. The Ingenuity Pathway Analysis (Qiagen, Redwood City, CA) package was used to identify canonical pathways, signaling cascades and other networks for the top 2,000 most statistically significant functionally related genes that may have been affected after ADT and/or vortioxetine treatment.
In vitro prostate cancer cell growth assays.
LNCaP (androgen-dependent, ATCC® CRL-1740™), PC-3 (androgen-independent, ATCC® CRL-1435™) and DU145 (androgen-independent, ATCC® HTB-81™) human prostate cancer cell lines were used. LNCaP and PC-3 prostate cancer cell lines were cultured in RPMI 1640 media (Sigma/ Corning™) with 10% FBS (Corning™ Fetal Bovine Serum - FBS Premium Cellgro) and 50 μg/ml gentamicin (Gibco, Life Technologies). DU145 prostate cancer cells were grown in IMEM media (ThermoFisher, Gibco Cell Culture) with 10% FBS (Corning™ Fetal Bovine Serum - FBS Premium Cellgro) and 25 μg/ml gentamicin (Gibco, Life Technologies).
The anti-proliferative potencies of vortioxetine, the androgen receptor (AR) antagonist enzalutamide, and the combination of vortioxetine with enzalutamide were evaluated using the sulforhodamine B (SRB) assay (Skehan et al. 1990). Treatments were performed in triplicate in a 96-well plate format with approximately 6,000 cells/well for LNCaP and 4,000 cells/well for PC-3 and DU145 prostate cancer cell lines, respectively. For combination treatments, at the time of drug addition, cell culture media was replaced with media (200 μl/well) containing either 1 μM vortioxetine or DMSO vehicle at a final concentration of 0.005%. A volume of 1 μl was then added to individual wells from 200x enzalutamide stocks while 0.5% DMSO was added to vehicle-treated wells. The percent growth of drug-treated cells was calculated at each concentration as compared to vehicle-treated control wells 48 h after drug addition. Cytotoxic efficacy was determined by comparison to the density of cells at the time of drug addition. Data represent the mean ± SEM from 3–4 independent experiments per treatment condition.
Statistical analyses:
Prism (GraphPad, San Diego, USA) and Statistica (Tibco, Palo Alto, USA) were used for statistical analyses. For the AST, trials to criterion on the ED task were analyzed by 2-way ANOVA (Castration × Drug), unless otherwise stated. Pairwise comparisons to detect the source of significant effects were performed using the Holm-Sidak test. For the electrophysiological data, the mean response magnitude for each animal at each stimulus intensity was used to construct a group current-response curve, which were compared using an extra sum-of-squares F-test (Jett et al. 2015). For the in vitro experiments, concentration-response curves were generated by non-linear regression analysis. Enzalutamide and vortioxetine interactions were compared by a 2-way ANOVA followed by the Holm-Sidak multiple comparison test. Significance was determined at p<0.05.
Results:
Experiment 1: Effects of androgen deprivation and vortioxetine on extra-dimensional set-shifting
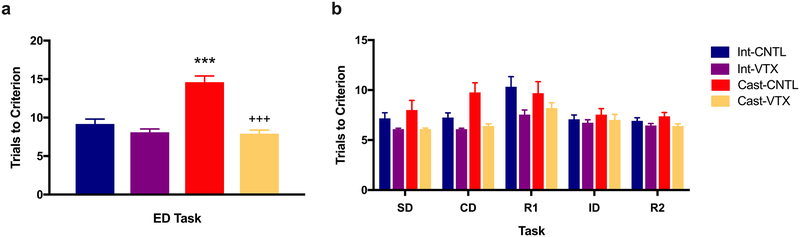
For this experiment, a total of 45 rats were used. Animals received chronic dietary vortioxetine (28 mg/kg/day) or control diet for 17 days through testing on the AST. On testing, there was a significant difference between the ID and ED stages in control animals, indicating formation of a cognitive set (t11 = 2.93, p<0.05). Two-way ANOVA for set-shifting performance on the ED task revealed significant main effects of both castration (F1,40 = 20.59, p<0.0001, n = 10–12/group), and drug (F1,40 = 35.36, p<0.0001), and a significant castration × drug interaction (F1,40 = 20.19, p<0.0001; Fig. 2a). Pairwise comparisons revealed that castration induced a deficit in set shifting (p<0.001). This deficit in cognitive flexibility was reversed in castrated rats treated with vortioxetine in the diet (p<0.001). Three-way ANOVA for all tasks preceding the ED set-shifting task revealed, as expected, a main effect of task (F4,40 = 9.31, p<0.000001), but no effect of castration (F1,40 = 2.19, p=0.15) and no significant interactions, suggesting that the detrimental effect of castration was specific to the set-shifting task. There was a main effect of drug alone on the tasks preceding ED (F1,40 = 21.62, p<0.0001; Fig. 2b). Likewise, there was an effect of vortioxetine alone on training (equivalent to the SD task; p<0.05), but again no effect of castration and no interaction.
Fig. 2.
Vortioxetine improves cognitive function on the AST and reverses deficits in set-shifting induced by castration. a Castrated male rats exhibited an impairment in cognitive set-shifting (***p<0.0001, castrated compared to intact controls), modeling the mPFC-dependent cognitive impairment seen after ADT. The impairment in set-shifting was reversed by chronic dietary vortioxetine treatment (2 weeks, 28 mg/kg/day) initiated 10 days post-castration (+++p<0.0001, castrated vortioxetine diet compared to castrated control diet). b Vortioxetine improved performance in tasks preceding the set-shifting task (p<0.0001), but there was no effect of castration on these tasks. All data presented as mean ± SEM; n = 6–12 per group.
Experiment 2: Effects of vortioxetine and androgen deprivation on evoked responses in afferent inputs to the mPFC
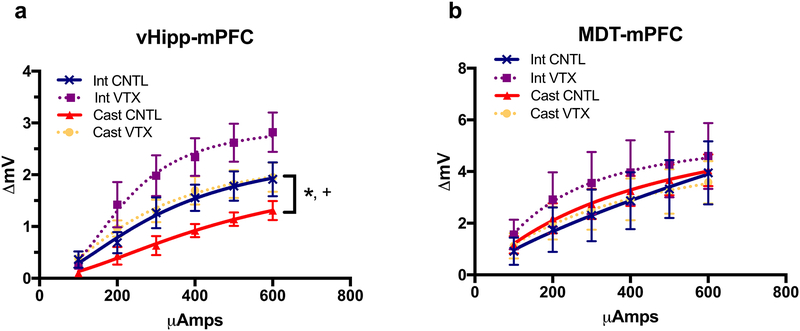
A total of 70 animals were used for both experiments (MDT and vHipp stimulation). Analyses of the current response curves revealed that the response of the mPFC to input from the vHipp was attenuated after androgen deprivation (F9, 162 =6.417, p<0.0001, Fig. 3a). Castrated animals that received vortioxetine had responses that were comparable to intact control rats fed control diet, and these responses were significantly increased from castrated animals receiving control diet (p<0.001). Chronic vortioxetine treatment in intact animals increased response in comparison to both intact controls and castrated vortioxetine-treated animals. By contrast, there was no change in responsivity of the mPFC to afferent input from the MDT across any groups (F9, 162=0.2105, p=0.9926, Fig. 3b).
Fig. 3.
Changes in responsivity of the mPFC to stimulation of excitatory afferent input from the vHipp but not the MDT after castration and vortioxetine. a Castrated male rats treated with control chow exhibited an attenuated electrical response in the mPFC evoked by stimulating the excitatory afferent input from the vHipp, compared to intact rats treated with control diet (*p<0.05). Chronic dietary vortioxetine (2 weeks, 28 mg/kg/day) normalized the evoked response of castrated male rats to a level comparable to that seen in intact control rats (+p<0.05). Note that vortioxetine alone increased the response in intact controls. n=6–8 per group. b There was no difference in electrical response in the mPFC evoked by stimulating the excitatory afferent input from the MDT. All data are presented as mean ± SEM, n=7–9 per group.
Experiment 3: Effects of androgen deprivation and vortioxetine on gene expression in the mPFC
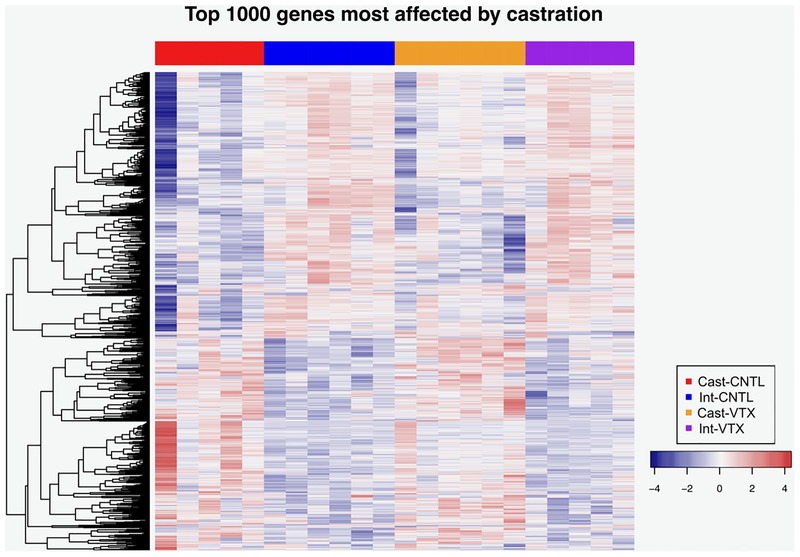
For the microarray analysis, 22 animals (5–6/group) were used to assess gene expression changes in the mPFC after each treatment condition. Transcripts were assessed for differential expression to test for main effects of castration or vortioxetine treatment in comparison to intact controls (Ritchie et al. 2015). Ingenuity pathway analysis (IPA ingenuity.com) was used to assess changes in expression of networks of genes associated with castration and/or vortioxetine treatment. In the analysis of main effects, 3,696 gene expression changes were associated with castration alone; 2,554 down-regulated and 1,142 up-regulated (false discovery rate <0.1; see Table 2A). A heatmap of expression profiles of the top 1,000 genes most affected by castration is shown in Fig. 4. Vortioxetine affected fewer genes than castration, and effects were less robust. Indeed, due to small sample size, the FDR for all individual genes affected by vortioxetine was >0.1. Therefore, with that caveat in mind, and using only p<0.05 as criterion, vortioxetine alone altered the expression of 482 genes, 377 increased and 105 decreased. After IPA analysis, gene pathways that were up- or down-regulated at rates higher than the background transcriptome (p-values < 0.01 and z-scores < −2.0 or > 2.0) are shown in Tables 2A–C. Pathways reflecting effects of castration and vortioxetine alone are shown in Tables 2A and 2B, respectively. In the interaction analysis, the FDR for all individual genes was >50%, again due to small sample size; the 34 genes differentially regulated in the interaction at p<0.025 are listed in table 2D. However, despite the high FDR for individual genes, several pathways were nonetheless differentially affected by castration in rats treated with vortioxetine vs. control diet (i.e., interaction effects at p-values < 0.01 and z-scores < −2.0 or > 2.0; Table 2C).
Table 2.
Altered gene expression patterns after castration and vortioxetine treatment. a–c Ingenuity Pathway Analysis of changes in expression of gene networks following castration and vortioxetine treatment. d Individual genes that were altered (p<0.025) in the interaction analysis. Genes are sorted by p value in ascending order.
| Table 2a – Main Effect of Castration | ||
|---|---|---|
| Canonical Pathway | z-score | Genes |
| Superpathway of Inositol Phosphate Compounds | −3.138 | FYN, NUDT9, PPFIA3, FLT3, NUDT12, PIK3R5, PLCH2, INPP5A, KLB, CDC25B, CD28, PLCE1, IP6K1, DUSP12, PTPRN, LOC103690006, TLR9, PLD4, ITPK1, DUSP1, ITPKC, DUSP23, MTMR7, PIK3CD, PPP5C, PIP4K2C, NUDT1 |
| RANK Signaling in Osteoclasts | −2.496 | MAP3K9, FLT3, MAP3K1, PIK3R5, MAP3K4, NFKB1, TLR9, MAPK11, KLB, XIAP, FOS, PIK3CD, MAP3K2, BIRC2 |
| Adrenomedullin signaling pathway | −2.449 | FLT3, TFAP2B, PIK3R5, NOTUM, KRAS, PRKG2, NFKB1, PLCH2, MAPK11, KLB, ARNT, KCNN1, PLCE1, GPR37, PRKAR1B, PPARG, TFAP2C, ITPR2, ADCY3, TLR9, FOS, KCNQ2, PIK3CD, TNF, NPR2 |
| Role of NFAT in Cardiac Hypertrophy | −2.4 | CACNA1I, PRKCQ, ITPR2, MAP3K1, FLT3, ADCY3, NOTUM, GNB5, PIK3R5, CSNK1A1, KRAS, TLR9, PLCH2, KLB, MAPK11, CABIN1, MEF2B, CTF1, CACNG3, PLCE1, IGF1, PRKAR1B, PIK3CD, RCAN2 |
| Glioblastoma Multiforme Signaling | −2.357 | CDKN2A, WNT10B, WNT9B, AXIN1, ITPR2, FLT3, NOTUM, PIK3R5, EGF, FZD9, KRAS, TLR9, PLCH2, KLB, FZD8, PLCE1, IGF1, SMO, PIK3CD, FZD7 |
| EGF Signaling | −2.333 | FOS, ITPR2, FLT3, MAP3K1, PIK3R5, EGF, PIK3CD, TLR9, KLB, MAPK11 |
| NGF Signaling | −2.324 | MAP3K9, CREBBP, MAP3K1, FLT3, PIK3R5, CRK, KRAS, MAP3K4, TLR9, NFKB1, KLB, SMPD2, SMPD4, TRAF4, PIK3CD, RPS6KA2, MAP3K2 |
| eNOS Signaling | −2.309 | PRKCQ, FLT1, ITPR2, FLT3, ADCY3, PIK3R5, SLC7A1, AQP8, TLR9, KLB, CHRM1, HSPA2, CNGB1, PRKAR1B, VEGFD, PIK3CD, MIP, CASP8, AQP2 |
| Colorectal Cancer Metastasis Signaling | −2.294 | LRP5, WNT10B, MMP28, WNT9B, GRK2, PTGER3, AXIN1, DVL1, ADCY3, FLT3, GNB5, PIK3R5, EGF, FZD9, KRAS, TLR9, NFKB1, KLB, TLR2, FOS, FZD8, PRKAR1B, SMO, VEGFD, PIK3CD, TNF, LRP1, FZD7 |
| 3-phosphoinositide Biosynthesis | −2.236 | FYN, NUDT9, NUDT12, PPFIA3, FLT3, LOC103690006, PIK3R5, TLR9, KLB, CDC25B, CD28, ITPK1, DUSP1, DUSP23, MTMR7, PIK3CD, DUSP12, PTPRN, PPP5C, PIP4K2C, NUDT1 |
| PKCθ Signaling in T Lymphocytes | −2.183 | CACNA1I, FYN, MAP3K9, PRKCQ, HLA-A, MAP3K1, FLT3, PIK3R5, KRAS, MAP3K4, TLR9, NFKB1, CD3D, KLB, CACNG3, FOS, CD28, POU2F1, ZAP70, PIK3CD, MAP3K2 |
| Renin-Angiotensin Signaling | −2.138 | PRKCQ, ITPR2, ADCY3, FLT3, MAP3K1, REN, PIK3R5, KRAS, NFKB1, TLR9, MAPK11, KLB, FOS, PRKAR1B, PIK3CD, TNF |
| Mouse Embryonic Stem Cell Pluripotency | −2 | AXIN1, CREBBP, FLT3, DVL1, PIK3R5, FZD9, KRAS, TLR9, MAPK11, KLB, XIAP, FZD8, BMPR1A, SMO, PIK3CD, DVL3, FZD7 |
| Table 2b - Main Effect of Vortioxetine | ||
|---|---|---|
| Canonical Pathway | z-score | Genes |
| Activation of IRF by Cytosolic Pattern Recognition Receptors | 2.309 | DHX58, NFKBID, IKBKG, IRF7, JUN, NFKBIA, CREBBP, MAPK10, STAT2, IKBKE, ADAR, TNF |
| NF-κB Signaling | 2.294 | MAP3K14, FLT1, PIK3C2A, MYD88, CREBBP, MAP3K1, FLT3, FLT4, IGF2R, TAB3, NFKBID, TRADD, IKBKG, IL18, NFKBIA, BMPR1A, ZAP70, MAP3K7, PRKACA, AKT3, PIK3CD, MAP3K8, TNFRSF1B, TNF |
| Opioid Signaling Pathway | 2.117 | RGS1, RGS18, CACNA2D2, GRIN2D, SRF, AP2A2, NFKBIA, CACNG7, AKT3, RPS6KA2, PRKCA, CACNA1G, CACNB4, CREBBP, RGS16, RGS4, PPP3CC, CREB5, CALM1 (includes others), FOS, PENK, LYN, PRKACA, RGS8, RPS6KA1, MAPK7, ELK1 |
| Endothelin-1 Signaling | −2.294 | PLA2G16, PIK3C2A, ABHD3, GNA11, FLT3, PLA2G1B, CASP4, PLD4, FOS, PLA2G4E, HMOX1, CASP6, JUN, PLCE1, GNA15, LCAT, CASP2, PLA2G4B, MAPK10, CASP1, PIK3CD, ECE1, MAPK7, PLCL1, PRKC |
| Osteoarthritis Pathway | −2.263 | GLI2, FRZB, SMAD3, WNT16, CASP4, FZD1, SDC4, HES1, PGF, VEGFA, CASP6, CTNNA2, CASR, ELF3, PRG4, FGF18, BMPR1A, FOXO3, CASP1, SMO, VEGFD, TNFRSF1B, S1PR2, IL1RAPL2, DDIT4, CREBBP, COL2A1, FZD9, CEBPB, CREB5, FZD8, FZD4, CASP2, SIRT1, TNF, PPARGC1A |
| Choline Biosynthesis III | −2 | PLD4, HMOX1, CHPT1, PCYT1A |
| Table 2c - Interaction: Differential Effects of Castration in Rats Receiving Vortioxetine and Control Diet | ||
|---|---|---|
| Canonical Pathway | z-score | Genes |
| Production of Nitric Oxide and Reactive Oxygen Species in Macrophages | 3.13 | PPARA, PPP1R14C, MAP3K9, PTPN6, APOM, PRKCQ, MAP3K6, TNFRSF1A, FLT3, PLCG1, JAK2, NFKB1, SAA4, SPI1, MAPK11, FOS, RHOQ, NCF2, MAP3K8, PRKCH, CHUK, TNFRSF1B, ATM |
| Type I Diabetes Mellitus Signaling | 2.887 | MYD88, TNFRSF1A, HLA-A, SOCS6, SOCS4, JAK2, NFKB1, CD3D, MAPK11, CHUK, CASP8, TNFRSF1B, FASLG, HLA-E |
| FAT10 Cancer Signaling Pathway | 2.53 | SMAD2, MAD2L1, TNFRSF1A, ACKR3, SMAD4, CHUK, ACVR2B, NFKB1, TNFRSF1B, ACVR1C |
| TNFR1 Signaling | 2.333 | FOS, CASP6, PAK6, TNFRSF1A, PAK2, CHUK, NFKB1, CASP8, BIRC2 |
| p38 MAPK Signaling | 2.309 | MYC, RPS6KB1, TIFA, MAPKAPK3, TNFRSF1A, CREB1, PLA2G4B, RPS6KA3, IRAK3, RPS6KA2, TNFRSF1B, ELK1, MAPK11, FASLG, MEF2B |
| Salvage Pathways of Pyrimidine Ribonucleotides | 2.138 | GRK4, MAP3K9, PRKCQ, MAP3K6, SGK1, APOBEC2, UPP1, GRK5, PRKX, PAK2, PRKAA2, MAP3K8, PRKCH, MAPK7 |
| Table 2d: Genes Altered in the Interaction of Castration and Vortioxetine | |||
|---|---|---|---|
| Gene | p value | Gene | p value |
| OLR278 | 0.001740572 | RPS6KA2 | 0.017149029 |
| RGL1 | 0.002514402 | PLIN4 | 0.018137806 |
| RIN1 | 0.003228111 | FBXW10 | 0.018673401 |
| CARD9 | 0.007659412 | BPIFA5 | 0.019528063 |
| CHAT | 0.008204031 | CAPG | 0.020347608 |
| CRABP1 | 0.008388713 | ICAM2 | 0.021024904 |
| CGN | 0.009744972 | GYPC | 0.022771414 |
| DHX58 | 0.010882921 | CLGN | 0.023074521 |
| RNF19A | 0.011218841 | CALCRL | 0.023213184 |
| COLGALT2 | 0.012662084 | KLF12 | 0.02335497 |
| PIGL | 0.013260137 | CLEC16A | 0.023388837 |
| ZSWIM4 | 0.014538369 | TMEM120A | 0.023567478 |
| ABCC2 | 0.014604031 | ARL4D | 0.023629671 |
| HCRTR1 | 0.016347204 | METTL11B | 0.023760717 |
| FAM163A | 0.016641643 | IL17RE | 0.02450042 |
| ENTPD3 | 0.0169542 | SERINC2 | 0.024658007 |
| TREX2 | 0.016987793 | NPR2 | 0.024998929 |
Fig. 4.
Heatmap of gene expression showing the 1,000 transcripts most significantly affected by castration (down-regulated in blue, up-regulated in red). Transcripts, shown in rows, were mean-centered, scaled, and ordered by hierarchical clustering. Columns represent individual samples, ordered by group.
Experiment 4: Effects of vortioxetine on androgen-dependent prostate cancer cell lines
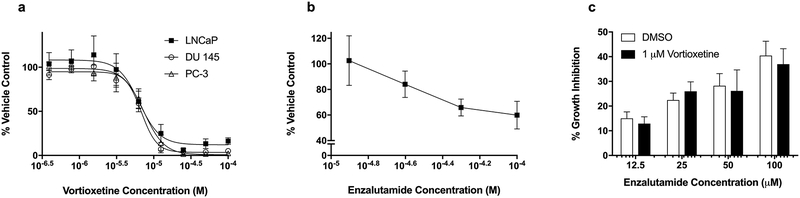
The effects of vortioxetine on the growth of LNCaP androgen-dependent prostate cancer cells, as well as DU145 and PC-3 androgen-independent prostate cancer cells, were evaluated using the sulforhodamine B assay. Concentrations lower than 3 μM vortioxetine did not affect the growth of any of these prostate cancer cell lines over a 48 hr period as compared to vehicle treated controls (Fig 5a). At higher vortioxetine concentrations, growth inhibition was observed with IC50 values of 6.8 – 7.3 μM and cytotoxic effects observed at concentrations greater than 10 μM. These results demonstrate that vortioxetine does not enhance the growth of prostate cancer cells in culture and that it actually inhibits their growth and promotes cytotoxicity at micromolar concentrations.
Fig. 5.
Vortioxetine does not increase the proliferation of or inhibit the anti-proliferative effects of androgen deprivation on prostate cancer cells in vitro. a Analysis of the concentration-dependent effects of vortioxetine on the growth of LNCaP, PC-3, and DU 145 prostate cancer cell lines over 48 h using the SRB assay indicate that vortioxetine did not promote cancer cell growth. Results are expressed as percent growth compared to vehicle controls (mean ± SEM; n=3). b Enzalutamide inhibited the growth of androgen-dependent LNCaP prostate cancer cells over 48 h in a concentration-dependent manner, evaluated using the SRB assay (n=4). C) Vortioxetine (1 μM) had no effect on the enzalutamide-dependent growth inhibition of LNCaP prostate cancer cells (n=3).
The antiproliferative effects of the AR antagonist enzalutamide on the androgen-dependent LNCaP prostate cancer cell line were evaluated. Enzalutamide caused a concentration-dependent inhibition of LNCaP proliferation over a range of 12.5 – 100 μM (Fig. 5b). Importantly, 1 μM vortioxetine, a concentration that did not affect the growth of these cells on its own, did not significantly impact the antiproliferative effects of enzalutamide (Fig. 5c).
Discussion:
Cognitive impairment induced by ADT can negatively impact the quality of life for prostate cancer survivors, their families and caregivers, and can deter patients from seeking or continuing successful treatment. In addition to the cognitive impairment that occurs after treatment, recent studies have shown that men who undergo ADT are predisposed to subsequent diagnoses of Alzheimer’s and dementia (Nead et al. 2016; Nead et al. 2017). Further, it has been reported that older cancer patients (breast, prostate, and colorectal) with cognitive impairment at baseline had an increased likelihood of death compared to cognitively intact counterparts (Libert et al. 2016). Therefore, improving cognitive capability may even improve treatment outcome.
Our lab has used the AST to assess cognitive set-shifting, a form of mPFC-dependent cognitive flexibility in rodents, under conditions that compromise the function of this brain region. We have demonstrated deficits in cognitive flexibility after chronic stress, effectively modeling the cognitive impairment in stress-related neuropsychiatric disorders such as depression and post-traumatic stress disorder (Bondi et al. 2010; Bondi et al. 2008). In this context, we have also demonstrated the efficacy of traditional (e.g., SSRI and SNRI anti-depressants) and novel (e.g., ketamine) therapeutic interventions, including vortioxetine (Fucich et al. 2016; Jett and Morilak 2013; Lapiz and Morilak 2006; Wallace et al. 2014). Thus, the AST as a model of mPFC-dependent cognition is sensitive both to manipulations that induce pathology, and to relevant and effective therapeutic interventions. In the present study, castration impaired cognitive flexibility on the set-shifting task, reflecting the deficits in mPFC-mediated executive function that have been reported in prostate cancer patients after ADT. This is also consistent with neuroimaging studies of prostate cancer patients showing that the mPFC is hypoactive after ADT (Chao et al. 2012). Chronic dietary vortioxetine treatment restored set-shifting in castrated male rats. It is worth noting that vortioxetine also improved performance on tasks preceding the ED set-shifting task, in the absence of any effect of castration on these tasks. Therefore, vortioxetine likely has positive effects across multiple brain regions. Nonetheless, castration selectively impaired mPFC-mediated set-shifting, and vortioxetine reversed this effect, without significant effect in intact controls. Thus, these results suggest that vortioxetine may be potentially beneficial in mitigating cognitive decline associated with ADT treatment in prostate cancer patients.
Vortioxetine is an FDA-approved antidepressant that has been shown to improve cognitive impairment in both preclinical models (Sanchez et al. 2015; Wallace et al. 2014) and in depressed patients (Katona et al. 2012; Mahableshwarkar et al. 2015; McIntyre et al. 2016; McIntyre et al. 2014). In addition to blocking the serotonin transporter, vortioxetine interacts selectively with several other serotonin receptors that are thought to give it additional efficacy in mitigating cognitive impairment. Vortioxetine is an agonist at the 5-HT1A receptor, partial agonist at the 5-HT1B receptor, and antagonist at the 5-HT1D, 5-HT3A, and 5-HT7 receptors (Bang-Anderson et al. 2011). The potential contributions of these receptor interactions to overcoming cognitive deficits in the context of stress and depression have been discussed in Wallace et al. (2014). Clinical studies have shown that the beneficial effects of vortioxetine on cognition are independent of its antidepressant effects (McIntyre et al. 2014). Nonetheless, vortioxetine is an effective antidepressant, and while depression may not be a factor in our rodent model of ADT, alleviating the symptoms of depression, which is prevalent within prostate cancer patients (Pirl et al. 2002; Watts et al. 2014), may offer an additional potential benefit toward improving quality of life in prostate cancer patients.
We investigated circuit-level mechanisms that may underlie changes in cognition induced by ADT and vortioxetine by assessing functional changes in the response of the mPFC to excitatory afferent input. The mPFC response to activation of the afferent pathway from the vHipp was reduced in castrated rats, and the attenuated response in castrated rats was restored by vortioxetine treatment. These effects were specific to the vHipp-mPFC afferent pathway, as there were no changes in response of the mPFC to stimulation of the afferent from the MDT. This pathway specificity may have been conferred by differential expression of the androgen receptor (AR), which is much higher in the cortex and Hipp than in the medial thalamus (Simerly et al. 1990). The CA1 region of the Hipp is among the regions with the highest density of ARs in the rodent brain (Kerr et al. 1995). The same study found that AR mRNA expression was significantly decreased in surgically castrated and intact rats treated with the AR antagonist, flutamide. This indicates that the Hipp is a crucial site for androgen-mediated modulation of brain function, and highlights the possibility that the detrimental effects of ADT may originate within local hippocampal circuitry, thereby reducing synaptic efficacy of hippocampal input to target regions such as the mPFC. Given that vortioxetine increased responsivity in the vHipp-mPFC pathway of intact control animals, our results support other preclinical results indicating that vortioxetine can upregulate plasticity-related mechanisms in the mPFC. This may reveal a potential mechanism by which vortioxetine overcame changes in cognition induced by androgen depletion.
Androgens have a wide range of effects on brain structure and function, and ARs are found throughout the brain (Simerly et al. 1990). Mechanisms underlying successful performance on cognitive tasks such as set-shifting and visuospatial memory are likely to require activity-dependent morphological and functional plasticity in neuronal circuits and synapses in the mPFC, Hipp, and the pathways connecting them. The AR is a transcription factor that regulates gene expression, the activation of which has been shown to promote structural plasticity and dendritic spine remodeling, and to increase synaptogenesis (Hajszan et al. 2007; Hajszan et al. 2008; Hawley et al. 2013; Leranth et al. 2003; MacLusky et al. 2006). These findings suggest that androgen depletion can have lasting dysregulatory effects on plasticity-related processes that can lead to changes in synaptic efficacy and compromise cognitive function. Vortioxetine has also been shown to enhance neuroplasticity by promoting dendritic elaboration and spine maturation (Chen et al. 2016). Preclinical studies in rodents indicate that vortioxetine can alter plasticity-related gene expression in the frontal cortex, Hipp and amygdala (du Jardin et al. 2016; Kugathasan et al. 2016; Li et al. 2015; Waller et al. 2017). Therefore, both the detrimental effects of ADT and the beneficial effects of intervention with vortioxetine on cognition might be accompanied by changes in gene expression that regulate neuronal plasticity.
Our microarray data were consistent with these observations, as castration down-regulated the expression of numerous genes and pathways. While vortioxetine affected fewer genes and pathways than castration, it also up-regulated pathways involved in neuronal communication, neuroplasticity and neuroinflammation (e.g., opioid signaling, endothelin-1 signaling, NF-kB signaling). Other pathways that were differentially down-regulated by castration in rats receiving control diet but not in rats receiving vortioxetine treatment are also implicated in neuroplasticity and remodeling, as well as neuroinflammation and neurodegeneration (e.g., TNFR1 signaling, p38 MAPK signaling) (Becker et al. 2015; Correa and Eales 2012). From the pathway analysis, we plan in future studies to examine changes in the expression of individual candidate genes that are shared by differentially affected pathways, or that are specifically implicated in processes related to neuronal plasticity and dendritic remodeling, including factors such as ribosomal protein S6 kinases, CREB, fos, PAK, and MAPK. These factors all have well-documented roles in plasticity-related processes, making them potential candidates for a role in the mechanisms by which androgen deprivation may alter the response of mPFC to afferent input and compromise cognitive function, and by which vortioxetine may overcome such effects. Other important molecules that were highlighted from our interaction analysis of individual genes encode proteins involved in cell adhesion, trafficking, and migration, including genes such as CGN, ICAM2, and ARLD4, to name a few. While these gene families have broad actions, the disruption of pathways in which they are implicated may induce significant changes in plasticity-related processes within the mPFC after castration. It is also important to point out, however, that an effective therapeutic intervention may not necessarily target the same mechanisms that were altered by the initial pathological process, but may instead initiate other processes to compensate for or overcome the initial deficit. Thus, it will be important to examine relevant candidate factors affected by castration and vortioxetine alone, as well as those differentially regulated by both.
Mitigating the detrimental and often debilitating side effects of cancer treatment is important for maintaining quality of life for cancer patients, and may increase survival by increasing the likelihood of initiating or continuing effective treatment. It is also essential, however, that any approach aimed at improving the side effects of cancer treatment should not exacerbate the cancer nor interfere with the anti-cancer efficacy of the primary treatment. If the present results are to suggest that vortioxetine may be useful in the management of cognitive impairment after ADT, it was especially important to test its effects on prostate cancer cells. Vortioxetine is a partial agonist at the 5-HT1B receptor and a full agonist at the 5-HT1A receptor. Serotonin receptors are expressed on prostate cancer cells, and 5-HT1A and 5-HT1B antagonists have been shown to induce apoptosis and inhibit prostate cancer cell proliferation (Siddiqui et al. 2006). In the present experiment, we found that vortioxetine had no effect on the growth of either androgen-dependent or androgen-independent prostate cancer cells in vitro, and it was in fact cytotoxic at high concentrations. Vortioxetine also did not interfere with the anti-proliferative effects of the AR blocker, enzalutamide. Caution must be applied to this conclusion until the findings can be confirmed in in vivo assays, but the results suggest that vortioxetine would not directly exacerbate the growth of prostate cancer cells, nor impede the effectiveness of ADT in treating prostate cancer.
Several approaches are used to produce androgen deprivation clinically, including physical castration, androgen receptor antagonists, 5-α-reductase inhibitors, and gonadotropin releasing hormone (GnRH) agonists or antagonists. These approaches all ultimately achieve the same goal, to lower testosterone levels, and each present different advantages and disadvantages. Clinical studies have explored the physiological side effects that occur with ADT in prostate cancer, including changes in cardiovascular function, bone density, and metabolism (Gupta et al. 2017; Russell and Grossmann 2018), but the mechanisms underlying cognitive impairment associated with ADT have not been well studied. Cognitive impairment has been reported after multiple ADT approaches for the treatment of prostate cancer (Cherrier et al. 2009; Green et al. 2002; Nelson et al. 2008), suggesting that the cognitive changes are attributable to reduced testosterone activity rather than the specific method used to induce androgen deprivation. For the purpose of the current studies, we used surgical castration because it allowed the most rapid and complete depletion of androgen. This is not the method most commonly used in the clinic, a limitation of the present study, but it also minimized several potential confounds related, for example, to transient or compensatory increases in testosterone levels or changes in AR sensitivity. The cognitive impact of other methods to induce ADT in rodent models must also be explored to fully investigate the potential utility of drugs such as vortioxetine in mitigating cognitive impairment induced by ADT. Another limitation of this study was the absence of prostate cancer in the rats that underwent castration. Cancer itself can induce aberrant physiological processes involved in pain, metabolism, inflammation, and stress that can alter brain function both directly and indirectly, and may interact with the effects of ADT and vortioxetine. Such factors will also be addressed in future studies.
Conclusions:
The experiments presented here reveal that physical castration in adult male Sprague-Dawley rats induced a significant deficit in cognitive set-shifting on the AST, reflecting the deficits in mPFC-mediated executive function reported in prostate cancer patients after ADT. Chronic dietary vortioxetine treatment restored set-shifting performance in castrated male rats. Castration attenuated the local field potential response evoked by stimulation of the vHipp-mPFC afferent pathway, but not the MDT-mPFC pathway, and vortioxetine reversed the attenuated response in castrated animals. Gene expression data suggest that pathways involved in plasticity may be important in the mechanisms by which castration impairs cognition, and also by which vortioxetine improves cognition after castration. Finally, vortioxetine did not alter the growth of prostate cancer cells in vitro, nor inhibit the anti-proliferative effects of androgen deprivation. Taken together, the results of this study indicate that vortioxetine may be useful in alleviating cognitive impairment in prostate cancer patients undergoing ADT, for which there are currently no treatments available. Identifying strategies to mitigate ADT-induced cognitive impairment would substantively improve the quality of life for prostate cancer survivors.
Acknowledgements:
The authors thank Dr. Sarah Bulin for assistance with the electrophysiological experiments.
Funding: This work was supported by research grant RP180055 from the Cancer Prevention and Research Institute of Texas; research grant R01 CA224672 from the National Cancer Institute, National Institutes of Health; the Quincy and Estine Lee Endowment Fund; and pilot funding provided by the Mays Cancer Center, UT Health San Antonio. Gene expression data were generated by the Mays Cancer Center Genomics Shared Resource Facility (P30 CA054174). In-kind support was provided by H. Lundbeck A/S, which generously provided the drug-containing chow and control chow.
Abbreviations:
- ADT
androgen deprivation therapy
- AR
androgen receptor
- AST
Attentional Set-Shifting Test
- ED
extra dimensional
- FDA
Food and Drug Administration
- fMRI
functional magnetic resonance imaging
- Hipp
hippocampus
- vHipp
ventral hippocampus
- 5-HT
5-hydroxytryptamine
- MDT
medial dorsal thalamus
- mPFC
medial prefrontal cortex
- SRB
sulforhodamine B assay
- VTX
vortioxetine
Footnotes
Conflict of interest: Vortioxetine was provided by H. Lundbeck A/S, which had no input into the conduct of the study, analysis or interpretation of the data, and no role in the decision to publish or in the writing of the manuscript. Dr. Morilak receives research funding from Alkermes that has no relation to the present work. No other authors have any conflicts of interest related to the present work.
All research procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Health, San Antonio, and are compliant with ethical standards of the National Institutes of Health as specified in the Guide for the Care and Use of Laboratory Animals.
References:
- Bang-Anderson B et al. (2011) Discovery of 1-[2-(2,4-Dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): A novel multimodal compound for the treatment of major depressive disorder J Med Chem 54:3206–3221 [DOI] [PubMed] [Google Scholar]
- Becker D, Deller T, Vlachos A (2015) Tumor necrosis factor (TNF)-receptor 1 and 2 mediate homeostatic synaptic plasticity of denervated mouse dentate granule cells Scientific reports 5:12726 doi: 10.1038/srep12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Jett JD, Morilak DA (2010) Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1-adrenergic receptors in medial prefrontal cortex Prog Neuro-Psychopharmacol Biol Psychiatry 34:913–923 doi: 10.1016/j.pnpbp.2010.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA (2008) Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment Neuropsychopharmacology 33:320–331 doi: 10.1038/sj.npp.1301410 [DOI] [PubMed] [Google Scholar]
- Chao HH et al. (2013) Effects of androgen deprivation on cerebral morphometry in prostate cancer patients – an exploratory study PloS one 8:e72032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HH et al. (2012) Effects of androgen deprivation on brain function in prostate cancer patients – a prospective observational cohort analysis BMC Cancer 12:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, du Jardin KG, Waller JA, Sanchez C, Nyengaard JR, Wegener G (2016) Vortioxetine promotes early changes in dendritic morphology compared to fluoxetine in rat hippocampus Eur Neuropsychopharmacol 26:234–245 doi: 10.1016/j.euroneuro.2015.12.018 [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Aubin S, Higano CS (2009) Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer Psychooncology 18:237–247 doi: 10.1002/pon.1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa SA, Eales KL (2012) The Role of p38 MAPK and Its Substrates in Neuronal Plasticity and Neurodegenerative Disease Journal of signal transduction 2012:649079 doi: 10.1155/2012/649079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis LJ, Griffiths K (2000) Endocrine treatment in prostate cancer Seminars in surgical oncology 18:52–74 [DOI] [PubMed] [Google Scholar]
- du Jardin KG, Müller HK, Sanchez C, Wegener G, Elfving B (2016) A single dose of vortioxetine, but not ketamine or fluoxetine, increases plasticity-related gene expression in the rat frontal cortex Eur J Pharmacol 786:29–35 [DOI] [PubMed] [Google Scholar]
- Fucich EA, Paredes D, Morilak DA (2016) Therapeutic effects of extinction learning as a model of exposure therapy in rats Neuropsychopharmacology 41:3092–3102 doi: 10.1038/npp.2016.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SM, Kuo YF, Shahinian VB (2011) Prevalent and incident use of androgen deprivation therapy among men with prostate cancer in the United States Urologic oncology 29:647–653 doi: 10.1016/j.urolonc.2009.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez BD et al. (2015) Course and predictors of cognitive function in patients with prostate cancer receiving androgen-deprivation therapy: A controlled comparison J Clin Oncol 33:2021–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green HJ et al. (2002) Altered cognitive function in men treated for prostate cancer with luteinizing hormone-releasing hormone analogues and cyproterone acetate: A randomized controlled trial BJU International 90:427–432 [DOI] [PubMed] [Google Scholar]
- Gupta D, Salmane C, Slovin S, Steingart RM (2017) Cardiovascular Complications of Androgen Deprivation Therapy for Prostate Cancer Current treatment options in cardiovascular medicine 19:61 doi: 10.1007/s11936-017-0563-1 [DOI] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Johansen JA, Jordan CL, Leranth C (2007) Effects of androgens and estradiol on spine synapse formation in the prefrontal cortex of normal and testicular feminization mutant male rats Endocrinology 148:1963–1967 doi: 10.1210/en.2006-1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C (2008) Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas Horm & Behav 53:638–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley WR, Grissom EM, Martin RC, Halmos MB, Bart CL, Dohanich GP (2013) Testosterone modulates spatial recognition memory in male rats Hormones and behavior 63:559–565 doi: 10.1016/j.yhbeh.2013.02.007 [DOI] [PubMed] [Google Scholar]
- Jamadar RJ, Winters MJ, Maki PM (2012) Cognitive changes associated with ADT: a review of the literature Asian journal of andrology 14:232–238 doi: 10.1038/aja.2011.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins VA, Bloomfield DJ, Shilling VM, Edginton TL (2005) Does neoadjuvant hormone therapy for early prostate cancer affect cognition? Results from a pilot study BJU Int 96:48–53 doi: 10.1111/j.1464-410X.2005.05565.x [DOI] [PubMed] [Google Scholar]
- Jett JD, Boley AM, Girotti M, Shah A, Lodge DJ, Morilak DA (2015) Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway Psychopharmacology 232:3123–3133 doi: 10.1007/s00213-015-3957-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett JD, Bulin SE, Hatherall LC, McCartney CM, Morilak DA (2017) Deficits in cognitive flexibility induced by chronic unpredictable stress are associated with impaired glutamate neurotransmission in the rat medial prefrontal cortex Neuroscience 346:284–297 doi: 10.1016/j.neuroscience.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett JD, Morilak DA (2013) Too much of a good thing: Blocking noradrenergic facilitation in medial prefrontal cortex prevents the detrimental effects of chronic stress on cognition Neuropsychopharmacology 38:585–595 doi: 10.1038/npp.2012.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona C, Hansen T, Olsen CK (2012) A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder Int Clin Psychopharmacol 27:215–223 [DOI] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ (1995) Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus Endocrinology 136:3213–3221 doi: 10.1210/endo.136.8.7628354 [DOI] [PubMed] [Google Scholar]
- Kugathasan P et al. (2016) In vivo and in vitro effects of vortioxetine on molecules associated with neuroplasticity J Psychopharmacol in press [DOI] [PubMed] [Google Scholar]
- Lapiz MDS, Morilak DA (2006) Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability Neuroscience 137:1039–1049 doi: 10.1016/j.neuroscience.2005.09.031 [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ (2003) Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats The Journal of neuroscience : the official journal of the Society for Neuroscience 23:1588–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Abdourahman A, Tamm JA, Pehrson AL, Sanchez C, Gulinello M (2015) Reversal of age-associated cognitive deficits is accompanied by increased plasticity-related gene expression after chronic antidepressant administration in middle-aged mice Pharm Biochem Behav 135:70–82 [DOI] [PubMed] [Google Scholar]
- Libert Y et al. (2016) Vulnerabilities in Older Patients when Cancer Treatment is Initiated: Does a Cognitive Impairment Impact the Two-Year Survival? PloS one 11:e0159734 doi: 10.1371/journal.pone.0159734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Prange-Kiel J, Leranth C (2006) Androgen modulation of hippocampal synaptic plasticity Neuroscience 138:957–965 [DOI] [PubMed] [Google Scholar]
- Mahableshwarkar AR, Zajecka J, Jacobson W, Chen Y, Keefe RSE (2015) A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder Neuropsychopharmacology 40:2025–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Harrison J, Loft H, Jacobson W, Olsen CK (2016) The effects of vortioxetine on cognitive function in patients with major depressive disorder: A meta-analysis of three randomized controlled trials Int J Neuropsychopharmacol 19:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Lophaven S, Olsen CK (2014) A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults Int J Neuropsychopharmacol 17:1557–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nead KT, Gaskin G, Chester C, Swisher-McClure S, Dudley JT, Leeper NJ, Shah NH (2016) Androgen Deprivation Therapy and Future Alzheimer’s Disease Risk J Clin Oncol 34:566–571 doi: 10.1200/jco.2015.63.6266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nead KT, Gaskin G, Chester C, Swisher-McClure S, Leeper NJ, Shah NH (2017) Association Between Androgen Deprivation Therapy and Risk of Dementia JAMA oncology 3:49–55 doi: 10.1001/jamaoncol.2016.3662 [DOI] [PubMed] [Google Scholar]
- Nelson CJ, Lee JS, Gamboa MC, Roth AJ (2008) Cognitive effects of hormone therapy in men with prostate cancer Cancer 113:1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirl WF, Siegel GI, Goode MJ, Smith MR (2002) Depression in men receiving androgen deprivation therapy for prostate cancer: A pilot study Psycho-oncology 11:518–523 [DOI] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies Nuc Acids Res 43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell N, Grossmann M (2018) Management of bone and metabolic effects of androgen deprivation therapy Urologic oncology doi: 10.1016/j.urolonc.2018.10.007 [DOI] [PubMed] [Google Scholar]
- Sanchez C, Asin KE, Artigas F (2015) Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data Pharmacology & therapeutics 145:43–57 doi: 10.1016/j.pharmthera.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Sharp AM et al. (2017) Vortioxetine reverses cognitive impairment induced by castration as a model of androgen deprivation therapy for prostate cancer in male rats Neuroscience Meeting Planner Washington, D C: Society for Neuroscience Program; No. 520.03 [Google Scholar]
- Siddiqui EJ, Shabbir M, Mikhailidis DP, Thompson CS, Mumtaz FH (2006) The role of serotonin (5-hydroxytryptamine1A and 1B) receptors in prostate cancer cell proliferation The Journal of urology 176:1648–1653 doi: 10.1016/j.juro.2006.06.087 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018 CA: a cancer journal for clinicians 68:7–30 doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW (1990) Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study The Journal of comparative neurology 294:76–95 doi: 10.1002/cne.902940107 [DOI] [PubMed] [Google Scholar]
- Skehan P et al. (1990) New colorimetric cytotoxicity assay for anticancer-drug screening Journal of the National Cancer Institute 82:1107–1112 [DOI] [PubMed] [Google Scholar]
- Wallace A, Pehrson AL, Sánchez C, Morilak DA (2014) Vortioxetine restores reversal learning impaired by 5-HT depletion or chronic intermittent cold stress in rats Int J Neuropsychopharmacol 17:1695–1706 doi: 10.1017/S1461145714000571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller JA, Tamm JA, Abdourahman A, Pehrson AL, Li Y, Cajina M, Sanchez C (2017) Chronic vortioxetine treatment in rodents modulates gene expression of neurodevelopmental and plasticity markers Eur Neuropsychopharmacol 27:192–203 [DOI] [PubMed] [Google Scholar]
- Watts S, Leydon G, Birch B, Prescott P, Lai L, Eardley S, Lewith G (2014) Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates BMJ Open 4:e003901. [DOI] [PMC free article] [PubMed] [Google Scholar]