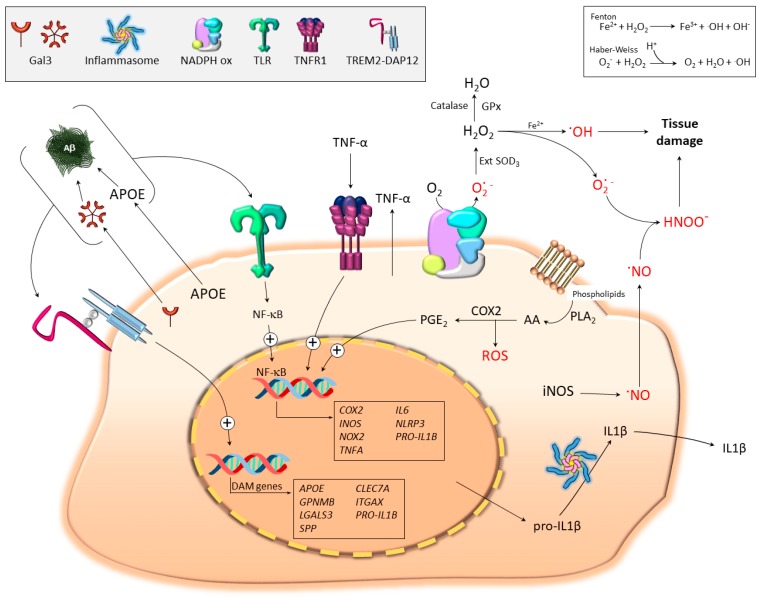
Figure 2.
Pro-oxidant microglia under disease conditions. TLR signaling drives NF-κB activation and transcription of proinflammatory and pro-oxidant molecules including iNOS, COX2, NADPH oxidase (NOX2), TNF-α, and pro-IL-1β. Assembly of NOX2 and ulterior activation constitutes an important source of superoxide anion and subsequent formation of radical oxygen (ROS) and nitrogen species. The figure illustrates how extracellular superoxide dismutates to H2O2 through the extracellular activity of superoxide dismutase (SOD) 3 and formation of hydroxyl radicals through the Fenton and Haber–Weiss reactions. Alternatively, superoxide anion may react with nitric oxide (NO) to form the highly toxic reactive peroxynitrites. The figure also illustrates the important role of COX2 in generating ROS. Thus, phospholipase A2 (PLA2) supplies arachidonic acid (AA) to COX2 for prostanoid biosynthesis (PGE2) along with ROS. NF-κB activation also leads to NLRP3 upregulation (the main inflammasome component). Upon appropriate stimulation (not shown; examples include K+ efflux or cathepsin release from damaged lysosomes), NLRP3 assembles a multiprotein platform resulting in caspase-1/caspase-8 activation and subsequent cleavage of pro-IL-1β into an active mature form (IL-1β). The figure also illustrates how different multivalent ligands, including Aβ, galectin-3 (GAL3), and APOE, may drive both TLR- and TREM2-signaling pathways. The switch from homeostatic to disease-associated microglia (DAM) is believed to be TREM2-dependent and it is accompanied by strong upregulation of different genes including GAL3 and APOE. These proteins can be released to the extracellular space, which together with Aβ and other DAMPS, may bind to and activate TLRs and trigger the microglia pro-oxidant response.