Abstract
Background
Prenatal inorganic arsenic (iAs) exposure is associated with pregnancy outcomes. Maternal capabilities of arsenic biotransformation and elimination may influence the susceptibility of arsenic toxicity. Therefore, we examined the determinants of arsenic metabolism of pregnant women in Bangladesh who are exposed to high levels of arsenic.
Methods
In a prospective birth cohort, we followed 1613 pregnant women in Bangladesh and collected urine samples at two prenatal visits: one at 4–16 weeks, and the second at 21–37 weeks of pregnancy. We measured major arsenic species in urine, including iAs (iAs%) and methylated forms. The proportions of each species over the sum of all arsenic species were used as biomarkers of arsenic methylation efficiency. We examined the difference in arsenic methylation using a paired t-test between first and second visits. Using linear regression, we examined determinants of arsenic metabolism, including age, BMI at enrollment, education, financial provider income, arsenic exposure level, and dietary folate and protein intake, adjusted for daily energy intake.
Results
Comparing visit 2 to visit 1, iAs% decreased 1.1% (p < 0.01), and creatinine-adjusted urinary arsenic level (U-As) increased 21% (95% CI: 15, 26%; p < 0.01). Drinking water arsenic concentration was positively associated with iAs% at both visits. When restricted to participants with higher adjusted urinary arsenic levels (adjusted U-As > 50 μg/g-creatinine) gestational age at measurement was strongly associated with DMA% (β = 0.38, p < 0.01) only at visit 1. Additionally, DMA% was negatively associated with daily protein intake (β = − 0.02, p < 0.01) at visit 1, adjusting for total energy intake and other covariates.
Conclusions
Our findings indicate that arsenic metabolism and adjusted U-As level increase during pregnancy. We have identified determinants of arsenic methylation efficiency at visit 1.
Keywords: Environmental arsenic exposure, Arsenic metabolism, Urinary arsenic metabolites, Pregnancy, Reproductive health
Introduction
Inorganic arsenic (iAs) is a ubiquitous, naturally occurring environmental toxicant [1, 2]. It has been linked to cancers [3] as well as increased risk of cardiovascular conditions [4], pregnancy complications [5], and developmental impairments [6]. iAs is a serious public health concern, as 200 million people worldwide are exposed to arsenic levels in drinking water that exceed the World Health Organization’s recommended limit of 10 μg/L [7].
Pregnant women and developing fetuses are especially susceptible to arsenic exposure. Arsenic-related adverse health conditions during pregnancy include anemia, nausea, vomiting, and abnormal cramping [5, 8]. Prenatal arsenic exposure is linked to reduced gestation time, low birth weight, spontaneous abortion, stillbirth, neonatal mortality, and infant mortality [9–17]. Identifying factors that influence susceptibility to arsenic toxicity in mothers and children can provide knowledge for risk assessment and guide effective interventions in underserved arsenic-endemic areas.
The human body metabolizes arsenic primarily in the liver. Ingested arsenate (iAsV) is reduced to arsenite (iAsIII) by arsenate reductase, reacts with glutathione, and is enzymatically methylated to monomethylarsonous acid (MMAIII) by the methyl-donor S-adenosyl methionine (SAM). MMAIII is either rapidly oxidized to monomethylarsonic acid (MMAV) or is involved in another cycle of methylation to form dimethylarsinous acid (DMAIII), which is then oxidized to dimethylarsinic acid (DMAV) [18]. Among organic forms of arsenic, trivalent forms (MMAIII and DMAIII) are highly toxic and are reactive intermediates in the arsenic methylation pathway, while pentavalent forms (MMAV and DMAV) are less toxic and are more readily excreted in urine [19]. The valence of organic arsenics are difficult to detect in human urine [20], but the proportions of urinary excretion of iAs, monomethyl forms of arsenic (MMA), and dimethyl forms of arsenic (DMA) are often used to evaluate arsenic methylation in vivo [21].
Maternal arsenic methylation efficiency is an important modifier of arsenic-related negative pregnancy outcomes. A lower proportion of DMA and a higher proportion of iAs may indicate decreased arsenic methylation, leading to increased iAs retention [22–25]. Epidemiological studies show that arsenic methylation efficiency can modify arsenic-related health risks in exposed pregnant women, as a greater proportion of iAs in urine is associated with impaired fetal growth [26, 27].
Many factors influence methylation of arsenic species, including arsenic exposure level, age, body mass index (BMI), sex, smoking, genetic factors, ethnicity, pregnancy, breastfeeding [28, 29], and genetic factors [30]. In addition, growing evidence suggest that nutrition influences arsenic metabolism and the risk of arsenic-related morbidity. Pregnant women undergo complex hormonal changes, which influence a remarkable increase in arsenic methylation during pregnancy [31–33]. Patterns and determinants of arsenic metabolism need to be studied separately in pregnant women not only because of their changed metabolism, but also because developing children are especially vulnerable to external toxicants [32, 34].
Arsenic exposure from drinking water is most severe in Bangladesh, where it is estimated that > 19 million people are exposed to > 5 times the standard arsenic level [35]. Therefore, we studied arsenic methylation and excretion profiles during pregnancy in a Bangladeshi cohort, as well as factors that may influence arsenic metabolism, including gestational weeks, water arsenic exposure, socioeconomic variables, dietary folate intake, and dietary protein intake. Together, these observations seek to provide a better understanding of how pregnancy affects arsenic metabolism.
Methods
Study population
During 2008–2011, we recruited 1613 pregnant women from two study sites in Bangladesh: one located in Sirajdikhan, a suburban upazila (subdistrict) located 29 km south of the capital city Dhaka; and the other in Pabna, a rural upazila located 122 km northwest from Dhaka [5, 36]. Eligible participants were adult women (≥18 years old) in their first trimester of pregnancy with a single fetus and who had a complete drinking water history that included tube well usage up to 6 months before pregnancy. These women participated in Dhaka Community Hospital’s (DCH) prenatal health program and planned to deliver at home with a DCH-trained midwife or at a DCH hospital or clinic. All participating families signed the informed consent of the study.
At enrollment at weeks 4–16 of pregnancy (visit 1), trained DCH healthcare workers who lived in the local area administered questionnaires to collect information on participants’ demographics, lifestyle, and medical condition. We collected a drinking water sample to assess arsenic exposure level, a urine sample to assess arsenic metabolite profile, and a toenail sample from each participant to assess long-term arsenic exposure levels. In addition, we provided all participants with a daily prenatal multivitamin with folate (400 μg) upon recruitment. Due to ethical responsibility, we advised participants to avoid using contaminated water sources.
The follow-up visit (visit 2) occurred at 21–37 weeks of pregnancy. Healthcare workers collected another urine sample and administered a locally validated food frequency questionnaire (FFQ) to acquire habitual dietary intake information from the previous 12 months [37]. At the time of visit 2, 1438 participants remained in the study. Reasons for loss-of-follow-up included loss of contact (N = 6), participant dropout (N = 18), miscarriage (N = 131), stillbirth (N = 11), sample failure (N = 2), missing samples (N = 2), and twin pregnancy (N = 5). After visit 2, we followed participants until one-month post-partum and collected their drinking water samples and toenail samples again to assess the stability of arsenic exposure.
Urinary arsenic metabolite concentration
For each participant, we collected spot urine samples at both visits 1 and 2 to assess concentrations of arsenic methylation species, including iAsIII, iAsV, MMA, and DMA. At the time of each visit, healthcare workers provided each participant a urine cup and instructed them how to obtain enough urine for analysis. Urine samples were sealed appropriately, brought to the local laboratory in iceboxes, and stored in a − 20 °C freezer. Frozen urine samples were shipped to the Department of Public Health, School of Medicine at Taipei Medical University, Taiwan, where they were stored in − 80 °C freezers. At the time of analysis, the urine samples were thawed at room temperature, sonicated for dispersion, filtered through a Sep-Pak C18 column (Mallinckrodt Baker Inc., NJ, USA), and transferred into 200-μL aliquots. We separated arsenic species fractions by high-performance liquid chromatography (Waters 501, Waters Associates, Milford, MA, USA) with columns from Phenomenex (Nucleosil, Torrance, CA, USA). Concentrations of the 4 urinary arsenic metabolites were determined by hydride generator atomic absorption spectrometry (PerkinElmer, Waltham, MA, USA). Standard reference material no. 2670a was obtained from National Institute of Standards and Technology (Gaithersburg, MD, USA), and recovery rates for iAsIII, iAsV, MMA, and DMA ranged 93.8–102.2%. The limit of detection (LOD) for each urinary arsenic metabolite was three times the standard deviation of 10 runs: 0.02 μg/L, 0.06 μg/L, 0.07 μg/L, and 0.10 μg/L for iAsIII, iAsV, MMA, and DMA, respectively [38]. Numbers of observations under the LOD were 810 (50%), 476 (30%), 316 (20%), and 1 (0%) for visit 1; and 841(58%), 374 (26%), 172 (12%), and 1 (0%) for visit 2, for iAsIII, iAsV, MMA, and DMA, respectively. We kept records below the LOD at their original value in our statistical analyses to provide the most information [39, 40]. To control for urine dilution, we analyzed the concentration of urinary creatinine using a colorimetric assay (Modular P800, Roche Inc., Mannheim, Germany).
Drinking water arsenic concentration
To assess drinking water arsenic exposure level (DW-As) and its stability during the follow-up period, two repeated drinking water samples were collected as previously described [5] at visit 1 and one-month post-partum. Briefly, tube well water samples were collected from the participant’s primary water source using well pumps after a one-minute purge. Samples were transferred to acid-washed polyethylene bottles and acidified with nitric acid for storage. Samples were then shipped to the laboratory (Environmental Laboratory Services, North Syracuse, NY, USA) and analyzed using a hybrid-generation technique of high-resolution inductively coupled process spectrometry (ICP-MS), following US Environmental Protection Agency method 200.8. Machines were sensitive to 1 μg/L of total arsenic concentration. Records with < 1 μg/L (N = 329, 20.5%) were assigned a value of 0.5 μg/L for statistical analyses (Kile et al. 2014).
Toenail samples
To assess long-term arsenic levels in the body, we collected and analyzed maternal toenail samples at visit 1 and at one-month post-partum as previously described [9, 41]. Briefly, toenail samples were digested with Optima nitric acid (Thermo Fisher Scientific, Waltham, MA, USA) and analyzed using ICP-MS for total toenail arsenic concentration (T-As) [42].
Dietary assessment
To obtain recalled food consumption frequency during the previous 12 months, we administered a locally-validated semi-quantitative FFQ at visit 2 [37]. We included 42 common food items in Bangladesh from five categories: [1] cereal and bread [2]; vegetables [3]; legumes, pulses, and seeds [4]; fish, poultry, meat, and eggs; and [5] milk-based food items. The scale of consumption frequency and portion sizes were as previously described [37]. Trained technicians entered FFQ data. Daily protein intake (g/day), folate intake (μg /day), and energy intake (kcal/day) were estimated using the 2013 Food Composition Table for Bangladesh [43]. For dish types that were not available in the questionnaire table, nutrient compositions were calculated based on average weighted recipes provided by local dietitians at DCH using nutrient retention factors and yield factors in the Food Composition Table.
Statistical analysis
We adjusted urinary arsenic metabolite concentrations (mg/g-creatinine) by absolute concentration (μg/L) divided by urinary creatinine concentration (mg/dL). Adjusted total urinary arsenic was the sum of adjusted urinary arsenic metabolites. The proportion of each urinary arsenic metabolite (iAsIII%, iAsV%, MMA%, and DMA%) was calculated using its concentration divided by the sum of all urinary arsenic metabolite concentrations. The proportion of iAs (iAs%) was the sum of iAsIII% and iAsV%.
We used Wilcoxon signed-rank test to compare the percentages of arsenic species in urine between two visits and paired t-tests to compare the log-transformed concentration of arsenic species, creatinine levels, and total urinary arsenic levels. We calculated Spearman correlations of urinary arsenic metabolites.
Linear regression models were used to examine potential determinants of arsenic metabolism biomarkers. iAs%, MMA%, and DMA% were treated as separate outcome variables. Independent variables were age, BMI at enrollment, arsenic exposure level (adjusted U-As, continuous), education level (<secondary education or ≥ secondary education), monthly income of the financial provider, daily protein intake, daily folate intake, and daily energy intake tertiles (low/medium/high). We fitted each visit and study site to a separate model. Based on previous literature, variables that are likely to be correlated with exposure and outcome were considered in models. Analysis was applied to all mothers, as well as to a restricted sample of participants with adjusted U-As of > 50 μg/g creatinine. We chose a cut-off point of 50 μg/g-creatinine because this level is around the 25th quantile of exposure (see Additional file 1: Table S1). Further, we excluded some outlying measurements from analysis (exclusion criteria: creatinine level < 10 mg/dL and adjusted U-As > 3000 μg/g-creatinine).
We separately compared the whole study population (N = 1613) and participants with no missing data (N = 1425 at visit 1; N = 1420 at visit 2). There were no significant differences in demographics or exposure level between the two study populations. All statistical analyses were performed with SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA).
Results
Most participants were Bangladeshi homemakers (99%), non-smokers (100%), did not chew tobacco or betel nut (99%), and reported physical activity during pregnancy as “on their feet all day but in a stationary position or only spend half the day moving around on their feet” (99%). Detailed information about participants’ demographics, lifestyle, and exposure information is shown in Table 1. Participants from Pabna had a higher average DW-As exposure level (79.5 ± 131.3 μg/L) compared to participants from Sirajdikhan (12.2 ± 47.4 μg/L). All participants reported taking prenatal vitamins with folate every day from visit 1 to visit 2. There were no significant differences in demographics or exposure level between the sample of all enrolled mothers and completed cases.
Table 1.
Characteristics of study participants in Sirajdikhan and Pabna upazilas, Bangladesh
| Sirajdikhan (N = 879) | Pabna (N = 727) | All mothers (N = 1176) | |
|---|---|---|---|
| Years of age, visit 1a | |||
| 22.8 ± 4.1 | 23.0 ± 4.2 | 22.9 ± 4.2 | |
| Education levelb | |||
| < Secondary education | 446 (51%) | 335 (46%) | 781 (49%) |
| ≥ Secondary education | 431 (49%) | 389 (54%) | 820 (51%) |
| BMI, visit 1 (kg/m2) | |||
| 21.0 ± 3.3 | 20.0 ± 2.9 | 21.0 ± 3.0 | |
| Financial provider’s monthly income (taka) | |||
| Unknown | 9 (1%) | 23 (3%) | 32 (2%) |
| 0–2000 | 5 (1%) | 10 (1%) | 15 (1%) |
| 2001–3000 | 39 (4%) | 197 (27%) | 236 (15%) |
| 3001–4000 | 178 (20%) | 222 (31%) | 400 (25%) |
| 4001–5000 | 328 (37%) | 167 (23%) | 495 (31%) |
| 5000–6000 | 190 (22%) | 63 (9%) | 253 (16%) |
| > 6000 | 129 (15%) | 44 (6%) | 173 (11%) |
| Gestational weeks, visit 1 | |||
| 11.1 ± 3.1 | 11.4 ± 3.0 | 11 ± 3.0 | |
| Gestational weeks, visit 2 | |||
| 28.6 ± 1.8 | 29.3 ± 1.9 | 28.9 ± 1.9 | |
| Environmental smoke exposure | |||
| No | 555 (63%) | 370 (51%) | 925 (58%) |
| Yes | 322 (37%) | 356 (49%) | 678 (42%) |
| Number of glasses of water drank per day | |||
| 7.7 ± 2.3 | 7.7 ± 2.4 | 8 ± 2.0 | |
| Drinking water arsenic exposure categories | |||
| Quartile 1: ≤0.89 μg/L | 309 (35%) | 92 (13%) | 401 (25%) |
| Quartile 2: 0.89–2 μg/L | 397 (45%) | 24 (3%) | 421 (26%) |
| Quartile 3: 2–33 μg/L | 103 (12%) | 278 (38%) | 381 (24%) |
| Quartile 4: > 33 μg/L | 69 (8%) | 332 (46%) | 401 (25%) |
| Drinking water arsenic (μg/L), visit 1 | |||
| 12.1 ± 47.4 | 79.5 ± 131 | 42.6 ± 101 | |
| Median | 1.4 | 27.0 | 2.0 |
| Drinking water arsenic (μg/L), one-month post-partumc | |||
| 6.8 ± 32.5 | 78.9 ± 133 | 42.7 ± 103 | |
| Median | 1.0 | 26.0 | 1.8 |
| Maternal toenail arsenic (μg/g), visit 1 | |||
| 2.2 ± 3.2 | 4.8 ± 5.9 | 3.4 ± 4.9 | |
| Median | 1.1 | 2.5 | 1.7 |
| Maternal toenail arsenic (μg/g), one-month post-partumd | |||
| 1.8 ± 3.6 | 3.6 ± 4.2 | 2.7 ± 4.0 | |
| Median | 0.7 | 2.0 | 1.2 |
| Daily dietary folate intake (μg) | |||
| 268 ± 96.7 | 396 ± 103 | 327 ± 118 | |
| Daily dietary protein intake (g) | |||
| 131 ± 47.9 | 219.3 ± 60.0 | 172 ± 69.6 | |
| Daily energy intake (kcal) | |||
| 3195 ± 978 | 3234 ± 763 | 3214 ± 885 | |
a Continuous variables are presented as mean ± standard deviation
b Categorical variables are presented as number of participants (percentage)
c Pearson’s correlation of drinking water arsenic concentration between the first visit and one-month post-partum is 0.72 (p < 0.0001)
d Pearson’s correlation of toenail arsenic concentration between the first visit and one-month post-partum is 0.84
Medians and 5th–95th percentiles of the proportions and concentrations of urinary arsenic metabolites at visit 1 and visit 2 are summarized in Table 2. Results of Wilcoxon signed-rank tests and paired t-tests of difference in each arsenic metabolite between the two visits are shown in Table 3. Median iAs% for all mothers decreased from 8.5% at visit 1 to 6.6% at visit 2 (mean change = − 1.1%; p < 0.01), mainly due to decreased iAsIII% (mean change = − 1.6%; p < 0.01). Median DMA% for all mothers increased from 85.7% at visit 1 to 87.9% at visit 2 (mean change = 1.1%; p < 0.01). No change was observed in MMA% for all mothers.
Table 2.
Urinary arsenic metabolites of study participants in Sirajdikhan and Pabna at visits 1 and 2
| Sirajdikhan | Pabna | All mothers | ||||
|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 1 | Visit 2 | Visit 1 | Visit 2 | |
| N | 879 | 783 | 727 | 662 | 1606 | 1445 |
| iAsIII%a | 0 (0, 12.6) | 0 (0, 9.9) | 3.1 (0, 17.8) | 0 (0, 11.5) | 0 (0, 15.9) | 0 (0, 10.6) |
| iAsV% | 1.1 (0, 20.9) | 1.8 (0, 26.2) | 3.8 (0, 18.6) | 4 (0, 20) | 2.1 (0, 19.6) | 2.6 (0, 23.6) |
| iAs% | 5.7 (0, 24.1) | 5.3 (0, 27.7) | 11.2 (1.4, 24) | 8 (0, 22.1) | 8.5 (0, 24) | 6.6 (0, 25.4) |
| MMA% | 3.5 (0, 13.4) | 4.3 (0, 11.8) | 6 (0.6, 13.1) | 5.6 (0.1, 11.3) | 4.9 (0, 13.2) | 4.8 (0, 11.6) |
| DMA% | 89.5 (66.9, 100) | 89.6 (66, 99.9) | 82 (66.2, 95.6) | 85.8 (68.9, 98) | 85.7 (66.6, 100) | 87.9 (67.3, 99.5) |
| iAsIII, μg/L | 0 (0, 8.8) | 0 (0, 7.7) | 1.7 (0, 57.9) | 0 (0, 27.8) | 0 (0, 28.9) | 0 (0, 16.7) |
| iAsV, μg/L | 0.2 (0, 8.8) | 0.5 (0, 12) | 2.5 (0, 36) | 2.1 (0, 51.9) | 0.6 (0, 21.8) | 0.9 (0, 22.5) |
| iAs, μg/L | 0.7 (0, 17.8) | 1.1 (0, 17.9) | 7.5 (0.2, 81.8) | 4.9 (0, 75.9) | 2.5 (0, 52.2) | 2.4 (0, 39.1) |
| MMA, μg/L | 0.5 (0, 10.6) | 1.1 (0, 11) | 4.1 (0.1, 42.5) | 3.4 (0, 35.1) | 1.3 (0, 30.3) | 1.7 (0, 21.5) |
| DMA, μg/L | 10.9 (1.9, 121) | 20.5 (3.6, 128) | 54.2 (8.8, 431) | 50.2 (7.4, 371) | 22.5 (2.6, 297) | 30 (4.5, 259) |
| U-As | 12.2 (2, 147) | 23.7 (4.3, 151) | 69.9 (9.7, 531) | 63.2 (8, 480) | 26.5 (2.9, 376) | 35.2 (5.1, 325) |
| U-creatinine, mg/dL | 23.1 (6, 113) | 34.6 (8.7, 126) | 41.4 (11.6, 181) | 35 (9.7, 117) | 30.3 (7.5, 159) | 34.9 (9.1, 123) |
| Adjusted iAsIII b | 0 (0, 14.9) | 0 (0, 10.9) | 3.5 (0, 67.6) | 0 (0, 50.5) | 0 (0, 48.3) | 0 (0, 27.7) |
| Adjusted iAsV | 0.6 (0, 33.4) | 1.3 (0, 36.5) | 5.5 (0, 98.1) | 6.1 (0, 123) | 1.8 (0, 67.5) | 2.5 (0, 77.4) |
| Adjusted iAs | 2.7 (0, 51.6) | 3.1 (0, 48.9) | 15.2 (0.8, 142) | 12.6 (0, 156) | 6.5 (0, 98.9) | 5.7 (0, 104) |
| Adjusted MMA | 1.7 (0, 36) | 2.6 (0, 24.4) | 8.2 (0.4, 76.6) | 8.3 (0.1, 72.5) | 3.8 (0, 57.9) | 4.3 (0, 52.5) |
| Adjusted DMA | 43.2 (13.8, 369) | 52.8 (21.2, 276) | 114 (30.1, 577) | 126 (39, 776) | 65.1 (17.5, 530) | 76.4 (24.8, 605) |
| Adjusted U-As | 48.6 (16.2, 470) | 61.6 (24.6, 338) | 139 (37.4, 784) | 154 (43.6, 972) | 77.5 (19.6, 677) | 90.4 (28.8, 770) |
a Proportions of urinary arsenic metabolites are presented as median and 5th–95th percentiles
b Concentrations of urinary arsenic metabolites are creatinine-adjusted (μg/g-creatinine) and presented as median and 5th–95th percentiles
Table 3.
Change in percentages of urinary arsenic metabolites and other arsenic biomarkers between two visitsa
| Sirajdikhan | Pabna | All mothers | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median change | Mean | p-valuea | Median change | Mean | p-value | Median change | Mean | p-value | |
| (5th–95th percentile) | change | (5th–95th percentile) | change | (5th–95th percentile) | change | ||||
| iAsIII%b | 0 (−10.6, 8.5) | −0.7 | < 0.01 | − 0.1 (− 15.7, 9) | −2.6 | < 0.01 | 0 (− 13.9, 8.7) | − 1.6 | < 0.01 |
| iAsV% | 0 (− 13.8, 18.3) | 0.8 | 0.09 | 0 (−14.2, 13.8) | 0.2 | 0.38 | 0 (− 14.2, 16.6) | 0.5 | 0.56 |
| iAs% | 0 (−16.3, 17.6) | 0.1 | 0.27 | −2.7 (−15.9, 12.3) | −2.5 | < 0.01 | −1.4 (− 16.2, 15.1) | −1.1 | < 0.01 |
| MMA% | 0.4 (−9.1, 9) | 0.5 | < 0.01 | −0.3 (−8, 5.7) | − 0.6 | < 0.01 | 0.1 (− 8.6, 7.3) | 0 | 0.91 |
| DMA% | −0.2 (−22.7, 21) | − 0.6 | 0.52 | 3.3 (−12.2, 20.6) | 3.1 | < 0.01 | 1.8 (−19.7, 20.8) | 1.1 | < 0.01 |
| log (unadjusted U-As)c | 0.6 (−1.8, 2.8) | 0.5 | < 0.01 | −0.1 (−2.2, 2) | − 0.1 | 0.03 | 0.3 (−2, 2.5) | 0.2 | < 0.01 |
| log(U-creatinine) | 0.3 (−1.7, 2.1) | 0.3 | < 0.01 | −0.2 (−2.1, 1.5) | − 0.3 | < 0.01 | 0.1 (− 1.9, 1.9) | 0.1 | 0.07 |
| log (adjusted U-As) | 0.6 (−1.8, 2.8) | 0.5 | < 0.01 | −0.1 (−2.2, 2) | − 0.1 | < 0.01 | 0.3 (− 2, 2.5) | 0.2 | < 0.01 |
| log (DW-As)d | 0 (−3.5, 2.5) | 0 | 0.89 | 0 (−1.3, 2) | 0.2 | < 0.01 | 0 (−2.7, 2.5) | 0.1 | 0.05 |
| log(T-As) | −0.4 (−1.8, 1.1) | − 0.4 | < 0.01 | − 0.3 (− 1.1, 0.7) | −0.3 | < 0.01 | − 0.4 (− 1.5, 0.8) | −0.4 | < 0.01 |
a The units of iAsIII%, iAsV%, iAs%, MMA% and DMA% are all “%”. The unit of unadjusted U-As, U-creatinine, adjusted U-As, DW-As and T-As are μg/L, mg/dL, μg/g-creatinine, μg/L and μg/g, respectively
b Wilcoxon signed rank test p-values for the first five variables, conditional on Sirajdikhan site (N = 782), Pabna site (N = 661), and all mothers (N = 1443)
c Paired t-tests of differences between visit 1 and visit 2, conditional on Sirajdikhan site (N = 782), Pabna site (N = 661), and all mothers (N = 1443)
d Paired t-tests of differences between visit 1 and one-month post-partum, conditional on Sirajdikhan site (N = 577), Pabna site (N = 573), and all mothers (N = 1150)
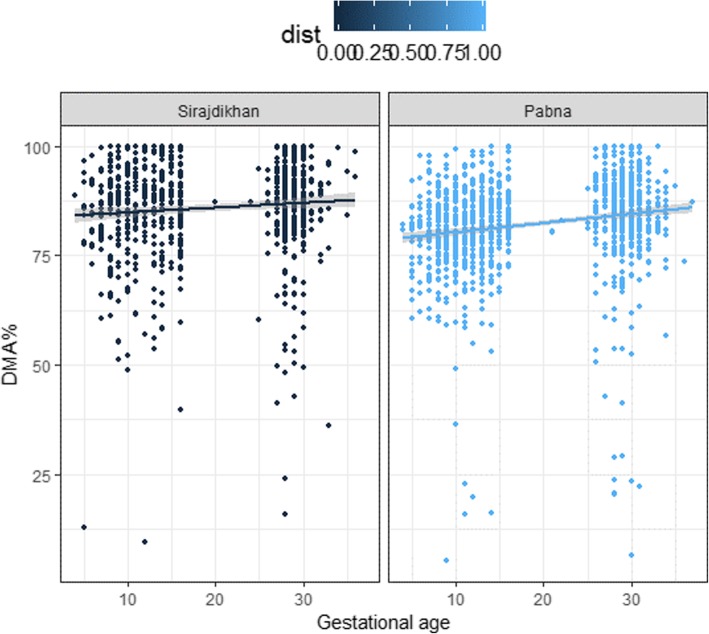
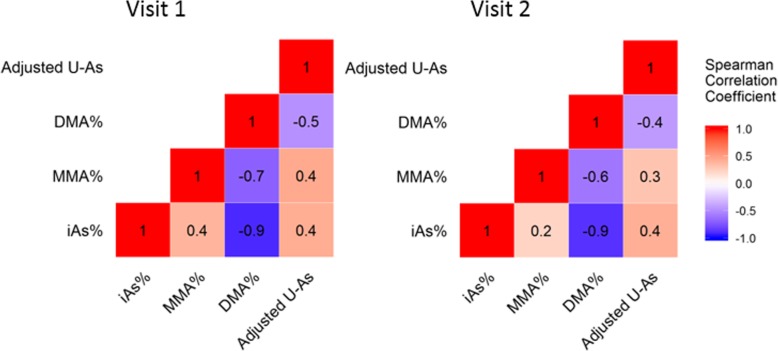
Restricting analysis to only participants with adjusted U-As of > 50 μg/g-creatinine, DMA% increased during the first trimester and remained high in the mid-to-late gestational period for participants in both Sirajdikhan and Pabna (Fig. 1). The significance of the increase would be shown with later regression analysis results. Data from all mothers are shown in Additional file 1: Figure S1 Further, proportions of arsenic metabolites in urine were highly correlated at visit 1 and visit 2 (Fig. 2). The adjusted U-As were correlated between visit 1 and visit 2 (see Additional file 1: Figure S2). Total urinary arsenic excretion was associated with urinary creatinine excretion (see Additional file 1: Figure S3). Thus, we used adjusted U-As in continuing analyses. Median U-As increased from 77.6 mg/g-creatinine at visit 1 to 90.4 mg/g-creatinine at visit 2. The mean difference of log-transformed U-As from visit 1 to visit 2 was 0.2 (p < 0.01), and the average ratio of U-As of visit 2 over visit 1 was 1.2. Log-transformed DW-As from visit 1 to one-month post-partum remained unchanged (p = 0.06; average ratio = 1.08). Log-transformed T-As decreased from visit 1 to one-month post-partum, with an average ratio of 0.70 (p < 0.01).
Fig. 1.
Scatter plots of DMA% and gestational weeks, restricted to participants with adjusted U-As > 50 μg/g-creatinine. [Figure legend: DMA% (%) plotted over gestational age (weeks), showing arsenic methylation efficiency of each participant at two repeated measurements. We applied a linear regression method to fit a solid line with the shade of standard error. We restricted exposure level to adjusted U-As > 50 μg/g-creatinine, which is ~25th percentile of adjusted U-As of both visits 1 and 2. Weeks of gestation was determined by ultrasound examination performed by trained healthcare workers. Dark blue dots indicate observations in Sirajdikhan; light blue dots indicate observations in Pabna]
Fig. 2.
Spearman correlation coefficients for arsenic metabolites at visit 1 and 2. [Figure legend: The unit of adjusted U-As is μg/g-creatinine, and the unit of iAs%, MMA% and DMA% are all “%”. All Spearman correlation coefficients had p < 0.01. The number of participants at visit 1 is 1605, while the number of participants at visit 2 is 1443]
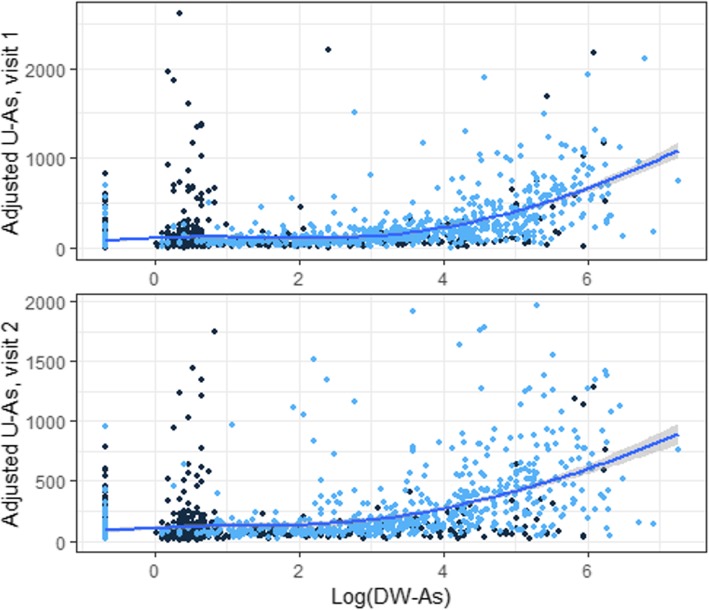
The association between adjusted U-As and DW-As is shown in Fig. 3. Because participants in Sirajdikhan had high U-As levels despite low DW-AS levels, they seemed to have other sources of arsenic exposure in addition to drinking water. We applied a linear model for dose-response relationship using data in Pabna, because participants in Pabna were less likely to be affected by other arsenic sources, resulting in a relationship of log (adjusted U-As) = 4.06 + 0.32 × log (DW-As). Table 4 shows determinants affecting the proportions of urinary arsenic metabolites at visit 1. When restricted to participants with adjusted U-As of > 50 μg/g-creatinine, gestational age at measurement was strongly associated with DMA% (β = 0.38, p < 0.01). Maternal age was positively associated with DMA% (β = 0.15, p = 0.04). Maternal higher education level was negatively associated with iAs% (β = − 1.16, p = 0.03). Additionally, DMA% was negatively associated with daily protein intake (β = − 0.02, p < 0.01). Every 100 g of daily dietary protein was associated with a 2% decrease of DMA in participants’ urine, adjusting for total energy intake and other covariates. Average iAs% for low, medium, and high tertiles of protein intake was 8.8, 9.3, and 11.8%, respectively. Average DMA% for low, medium, and high tertiles of protein intake was 85.2, 84.3, and 84.9%, respectively.
Fig. 3.
Scatter plot of adjusted U-As over log-transformed DW-As. [Figure legend: Adjusted U-As (unit of adjusted U-As: μg/g-creatinine) was plotted over log-transformed DW-As (unit of DW-As: μg/L), showing the correlation between environmental arsenic exposure and arsenic excretion. We applied Loess local polynomial linear regression to fit a solid curve line with the shade of standard error. Dark blue dots indicate observations in Sirajdikhan; light blue dots indicate observations in Pabna]
Table 4.
Determinants of the proportion of arsenic metabolites in urine during early gestation (visit 1)
| All mothers (N = 1595) | Restricted sample (adjusted U-As > 50 μg/g-creatinine; N = 1066)a |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| iAs% | MMA% | DMA% | iAs% | MMA% | DMA% | |||||||
| β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | |
| Intercept | 12.98 | < 0.01 | 9.09 | < 0.01 | 77.92 | < 0.01 | 12.01 | < 0.01 | 6.42 | < 0.01 | 81.57 | < 0.01 |
| Gestational age at measurement (week) | −0.13 | 0.21 | −0.26 | < 0.01 | 0.39 | < 0.01 | −0.17 | 0.03 | −0.21 | < 0.01 | 0.38 | < 0.01 |
| Maternal age (year) | −0.17 | 0.03 | −0.02 | 0.59 | 0.19 | 0.03 | −0.15 | 0.02 | 0 | 0.95 | 0.15 | 0.04 |
| BMI at enrollment (kg/m2) | 0.01 | 0.96 | −0.1 | 0.02 | 0.09 | 0.41 | −0.02 | 0.85 | −0.06 | 0.11 | 0.07 | 0.43 |
| Education level (≥secondary education) | −0.83 | 0.20 | 0.20 | 0.45 | 0.63 | 0.37 | −1.16 | 0.03 | 0.36 | 0.12 | 0.80 | 0.17 |
| Income of financial provider (≥3000 taka) | −0.68 | 0.30 | 0.52 | 0.05 | 0.16 | 0.82 | −0.68 | 0.20 | 0.07 | 0.76 | 0.61 | 0.31 |
| Adjusted U-As (μg/g-creatinine) | 0.01 | < 0.01 | 0.01 | < 0.01 | −0.01 | < 0.01 | 0.01 | < 0.01 | 0 | < 0.01 | −0.01 | < 0.01 |
| Daily protein intake (g) | 0.02 | 0.01 | 0 | 0.67 | −0.02 | 0.01 | 0.02 | < 0.01 | 0 | 0.47 | −0.02 | < 0.01 |
| Daily energy intake (kcal), medium tertile | 0.14 | 0.86 | −0.26 | 0.43 | 0.12 | 0.89 | −0.45 | 0.49 | 0 | 0.99 | 0.45 | 0.54 |
| Daily energy intake (kcal), high tertile | −2.30 | 0.02 | −0.39 | 0.32 | 2.69 | 0.01 | −2.53 | < 0.01 | −0.18 | 0.59 | 2.71 | < 0.01 |
| Daily folate intake (μg) | 0 | 0.75 | 0 | 0.07 | 0 | 0.71 | 0 | 0.56 | 0 | 0.06 | −0.01 | 0.21 |
a Linear models applied to all mothers, as well as to a restricted sample of participants with adjusted U-As > 50 μg/g creatinine. We selected this cut-off point because it is around the 25th percentile of the distribution of adjusted U-As at both visits. We did not include outliers of adjusted U-As in the analysis, which was > 3000 μg/g-creatinine because of very low creatinine level (< 10 mg/dL) (N = 11)
At visit 2, DMA% was only associated with adjusted U-As. We did not find other determinants of arsenic methylation efficiency at visit 2 (see Additional file 1: Table S2).
Discussion
Our findings suggest that arsenic methylation efficiency increases during pregnancy. At both visits, higher doses of arsenic were associated with increased iAs% and MMA%, as well as decreased DMA%, which is consistent with previous studies [44–46]. Further, other determinants affected arsenic methylation efficiency only in early gestational periods.
Our study included two study sites in Bangladesh, which were chosen for two reasons: they are under the service area of our collaborator, and they have different exposure levels of arsenic. Sirajdikhan has lower arsenic exposure levels, while Pabna has higher arsenic exposure levels. There were also differences in culture and dietary patterns between the two sites. A group of participants in Sirajdikhan had high levels of adjusted U-As despite low DW-As levels, indicating that these individuals were likely exposed to arsenic from their diets. For example, arsenic can accumulated in rice and rice is one of the common food of Bangladeshi in a daily basis [47, 48]. Participants may also exposed to organic forms of arsenic from sea foods [49]. The exposure misclassification may bias water arsenic’s true association between birth outcomes and developmental health outcomes. Spot urine arsenic level has limited ability to indicate long-term exposure, although it can be a good indicator of exposure from all sources including water.
Arsenic metabolism efficiency usually increases during pregnancy, particularly in the first trimester [31, 32]. Pregnant women usually have an ability to metabolize arsenic and excrete 70–100% of it as DMA in their urine, compared to an average of 60–80% for men and non-pregnant women [29]. We saw the same pattern in our cohort, especially when we restricted analysis to participants with higher arsenic exposure levels. DMA% increased during the first trimester and remained high in the mid-to-late gestational period in our cohort. From our separate linear regression analyses by study visits, arsenic methylation efficiency was associated with gestational age at visit 1 but not at visit 2. The physiological mechanism of these changes was not fully elucidated yet. Reduced iAsIII% possibly contributed to the decreased iAs%. However, as urine samples were not frozen immediately after collection, it is likely that some iAsIII was oxidized to iAsV. Thus, we focused our analysis on iAs%, which is not affected by oxidation or reduction.
Other than DMA%, U-As increased at follow-up visits, while DW-As remained unchanged. Toenail arsenic levels can represent the long-term internal dose of iAs over the past 3–6 months [19, 41]. Thus, the two measurements taken at visit 1 and one-month post-partum demonstrate relative arsenic absorption in the time periods before visit 1 through mid-to-late gestation. Our observation of decreased T-As may indicate increased arsenic clearance during pregnancy, although additional evidence is needed to confirm an increase in arsenic excretion. Previous studies hypothesize that increased U-As is partially due to increased DMA%, as DMAs are more readily excreted in urine [31, 32]. However, after excluding outliers of adjusted U-As, we found a negative association between ∆DMA% (DMA% difference between two visits) and ∆U-As (U-As difference between two visits) in linear regression (β = − 6.7, p < 0.01), adjusted for age, BMI, education, income, and DW-As. The underlying reason is unclear. It indicates that the improvements of arsenic methylation efficiency may not results in higher arsenic excretion.
Folate plays a key role in one-carbon metabolism, which promotes synthesis of the methyl donor SAM [50]. Several epidemiological studies showed that folate supplementation in folate-deficient cohorts were associated with improved arsenic methylation efficiency and arsenic elimination [51–53]. Our data show that average daily folate intake of participants was 327 μg. The majority of participants had insufficient daily folate intake compared to the recommended dose of 400–800 μg. We were not able to compare folate intake level at visit 1 and 2, because the estimate was based on a self-reported FFQ for the past year. We hypothesized that dietary folate intake was positively associated with arsenic methylation efficiency, but we did not find an association at either visit from regression analyses. At visit 1, participants had not yet received folate supplements, so diet was the main source of folate. This non-significant result about folate intake is in agreement with previous findings that folate levels during pregnancy marginally influence arsenic methylation [32, 34, 54]. At visit 2, every participant had been taking folate supplements, so there was less variation in participants’ folate levels and thus a reduced ability to show any effect. The average daily protein intake was 172 g for all mothers, which is much higher than the US daily recommendation of 75–100 g. Our method may overestimate the protein intake, but it represents the comparative protein intake among participants. Our study shows dietary intake of protein was associated with greater iAs% in the first trimester, which indicates reduced methylation efficiency, adding to the inconsistent literature regarding protein intake and arsenic-related effects. Steinmaus et al. investigated a US population and found that people in the lower quartile of protein intake excreted a higher proportion of ingested iAs as MMA and a lower proportion as DMA, compared to the upper quartile of protein intake [54]. Some human studies have found worse arsenic-associated health effects among those consuming lower amounts of meat, eggs, and vegetables [55, 56]. Kurzius-Spencer et al. found that higher protein intake was associated with a decreased proportion of iAs in urine [57]. However, Heck et al. found that higher intake of protein, methionine, and cysteine was associated with 10–15% greater total urinary arsenic excretion, after controlling for total energy intake, body weight, sex, age, tobacco use, and intake of other nutrients [58]. However, these studies did not properly address the complete biological role of protein intake. Inconsistent study results may be due to inaccurate measures or uncontrolled confounders. In addition, pregnant women compared with non-pregnant adults appear to have enhanced arsenic metabolism [34]. Therefore, factors affecting arsenic metabolism in non-pregnant women may not be applicable to our observations for pregnant women.
In addition, the association between DMA% and protein intake may be slightly confounded by some high-protein dishes, as Lin et al. found that toenail arsenic level is positively associated with consumption of fish and meat items [59]. The effect of dietary protein and folate intake may also be confounded by body size, as participants with different body sizes may proportionally consume less or more food. Nonetheless, our multiple linear regression models adjusted for BMI, and we found similar results in our analyses with and without adjusting for total energy intake.
In this study, we used proportions of arsenic metabolites in urine to represent arsenic methylation efficiency, which is commonly used in epidemiological studies. Ratios between arsenic metabolites were not used here, as many pregnant women have very low proportions of iAs and MMA, resulting in extreme values. We also found similar results in sensitivity analysis on adjusted iAs concentrations (μg/g-creatinine) and its determinants. Daily protein intake was positively associated with adjusted iAs concentration (p = 0.07), and the high tertile of daily energy intake had lower average iAs concentration (p = 0.69), adjusted for total urinary arsenic, age, BMI at enrollment, gestational age, education, and income of the financial provider.
Despite its strengths, our study also has several limitations. For instance, spot urine samples are only useful for reflecting short-term exposure. Although the percentage of arsenic metabolite is remarkably stable over time [21, 60], the time of day at sample collection may affect the observation of MMA% and iAs% [21, 61]. To minimize this confounding effect, healthcare workers in our study collected urine samples at a similar time of a day. Thus, while we did not control for bias from this confounder, it should not significantly bias the associations of arsenic methylation efficiency and its determinants. We observed good association between adjusted U-As and DW-As, indicating that adjusted U-As can be a reliable marker of exposure. Another limitation is that participants’ dietary intake was self-reported and thus subject to reporting bias, especially when we asked about diet over a long period of 12 months. Protein intake was very high compared to the US Dietary Reference Intake for pregnant women [62, 63]. However, FFQs may not accurately capture absolute dietary intake or active dose of nutrients, although FFQs have strong validity in correctly ranking nutrition intake compared to a food diary that does not rely on recall and memory [64]. To mitigate the effects of measurement errors in self-reported FFQs, we controlled for energy intake in regression analyses. Further, although we have food intake amount, we are not able to quantify arsenic in the diet, which could help capture other arsenic sources. Additionally, we only focused on dietary folate and protein intake but lack information on intake of other nutrients related to one-carbon metabolism, including cysteine, methionine, choline, and vitamin B-12 [65]. Future studies should investigate whether these nutrients affect arsenic methylation efficiency during pregnancy and whether nutritional supplementation is beneficial for arsenic detoxification.
This is the first study to evaluate the association between arsenic methylation efficiency and dietary intake of protein and folate in pregnant women. Patterns of arsenic methylation efficiency and total urinary excretion during pregnancy may vary by geographical area, due to variations in demographics, lifestyle, and dietary habits. Therefore, the difference in arsenic methylation efficiency between our two study centers is not fully understood.
Conclusions
Our findings suggest that both arsenic methylation efficiency and total urinary arsenic level increase during pregnancy in a cohort of women in Bangladesh. The patterns of arsenic methylation efficiency and total arsenic excretion varied by geographic area. Also, we found that greater dietary intake of protein or folate may not improve arsenic methylation efficiency. Future research should continue to study potential approaches to improve health outcomes in populations exposed to arsenic.
Supplementary information
Additional file 1: Table S1. Quantiles of adjusted urinary arsenic concentrations (U-As) (μg/g creatinine). Table S2. Determinants of the proportion of arsenic metabolites in urine in mid-to-late gestation (visit 2). Figure S1. Scatter plots of DMA% and gestational weeks for all mothers. Figure S2. Correlation between adjusted U-As at visit 1 and visit 2. Figure S3. Association between unadjusted U-As and urinary creatinine at visit 1 (top) and visit 2 (bottom).
Acknowledgements
The authors acknowledge the cooperation of study participants and community healthcare workers of Bangladesh for their contributions to this study. We thank the lab manager, Li Su, and crew at Harvard T.H. Chan School of Public Health, as well as Dr. Yu-Mei Hsueh and her lab at Taipei Medical University for their hard work processing urine samples.
Abbreviations
- BMI
Body mass index
- DCH
Dhaka Community Hospital
- DMA
Dimethyl forms of arsenic
- DMA%
Proportion of DMA in urine
- DMAIII
Dimethylarsinous acid
- DMAV
Dimethylarsinic acid
- DW-As
Drinking water arsenic level
- FFQ
Food frequency questionnaire
- iAs
Inorganic arsenic
- iAs%
Proportion of iAs in urine
- iAsIII
Arsenite
- iAsV
Arsenate
- ICP-MS
Inductively coupled process spectrometry
- MMA
Monomethyl forms of arsenic
- MMA%
Proportion of MMA in urine
- MMAIII
Monomethylarsonous acid
- MMAV
Monomethylarsonic acid
- SAM
S-adenosyl methionine
- T-As
Toenail arsenic level
- U-As
Adjusted total urinary arsenic level
Authors’ contributions
DC, LS, GM, QQ, and MR designed and conducted the Bangladesh Reproductive Study (Project Jeebon). SG designed the study of urinary arsenic metabolites during pregnancy. YH and her team contributed to urine sample analysis. SG and PL conducted statistical analyses. SG drafted the manuscript, and all authors contributed to its contents. All authors read and approved the final manuscript.
Funding
The design of Project Jeebon and its collection, analysis, data interpretation and manuscript writing were supported by National Institute of Environmental Health Sciences (NIEHS) grant R01 ES015533. This work was also supported by CDC (NIOSH) award 5T42OH008416.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to institutional review board specifications (but are available from the corresponding author on reasonable request).
Ethics approval and consent to participate
All procedures were approved by Institutional Review Boards of both Harvard T.H. Chan School of Public Health and the Dhaka Community Hospital Trust. All participating families signed the informed consent of the study.
Consent for publication
Not applicable.
Competing interests
All authors of this study disclose that they do not have any actual or potential competing interests regarding the submitted article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shangzhi Gao, Email: shg778@mail.harvard.edu.
Pi-I Lin, Email: shg778@mail.harvard.edu.
Golam Mostofa, Email: mostofa07@gmail.com.
Quazi Quamruzzaman, Email: dch@bangla.net.bd.
Mahmudur Rahman, Email: msrahman39@gmail.com.
Mohammad Lutfar Rahman, Email: mlr782@mail.harvard.edu.
Li Su, Email: lisu@hsph.harvard.edu.
Yu-mei Hsueh, Email: ymhsueh@tmu.edu.tw.
Marc Weisskopf, Email: mweissko@hsph.harvard.edu.
Brent Coull, Email: bcoull@hsph.harvard.edu.
David Chistopher Christiani, Phone: 617-432-3323, Email: dchris@hsph.harvard.edu.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12940-019-0530-2.
References
- 1.Welch AH, A WS, Helsel DR, Focazio MJ. Arsenic in Ground-Water Resources of the United States: US Geological Survey: Fact Sheet; 2000. p. 063–00.
- 2.WHO . Arsenic in Drinking Water. 2007. [Google Scholar]
- 3.NRC NRC. Critical aspects of EPA's IRIS assessment of inorganic arsenic: interim report. Washington: The National Academies Press; 2013. [Google Scholar]
- 4.Navas-Acien A, Sharrett AR, Silbergeld EK, Schwartz BS, Nachman KE, Burke TA, et al. Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. Am J Epidemiol. 2005;162(11):1037–1049. doi: 10.1093/aje/kwi330. [DOI] [PubMed] [Google Scholar]
- 5.Kile ML, Rodrigues EG, Mazumdar M, Dobson CB, Diao N, Golam M, et al. A prospective cohort study of the association between drinking water arsenic exposure and self-reported maternal health symptoms during pregnancy in Bangladesh. Environ Health. 2014;13(1):29. doi: 10.1186/1476-069X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeSesso JM, Jacobson CF, Scialli AR, Farr CH, Holson JF. An assessment of the developmental toxicity of inorganic arsenic. Reprod Toxicol. 1998;12(4):385–433. doi: 10.1016/S0890-6238(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 7.Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, et al. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect. 2013;121(3):295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopenhayn C, Bush HM, Bingcang A, Hertz-Picciotto I. Association between arsenic exposure from drinking water and anemia during pregnancy. J Occup Environ Med. 2006;48(6):635–643. doi: 10.1097/01.jom.0000205457.44750.9f. [DOI] [PubMed] [Google Scholar]
- 9.Kile ML, Cardenas A, Rodrigues E, Mazumdar M, Dobson C, Golam M, et al. Estimating effects of arsenic exposure during pregnancy on perinatal outcomes in a Bangladeshi cohort. Epidemiology. 2016;27(2):173–181. doi: 10.1097/EDE.0000000000000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad SA, Sayed MH, Barua S, Khan MH, Faruquee MH, Jalil A, et al. Arsenic in drinking water and pregnancy outcomes. Environ Health Perspect. 2001;109(6):629–631. doi: 10.1289/ehp.01109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloom MS, Surdu S, Neamtiu IA, Gurzau ES. Maternal arsenic exposure and birth outcomes: a comprehensive review of the epidemiologic literature focused on drinking water. Int J Hyg Environ Health. 2014;217(7):709–719. doi: 10.1016/j.ijheh.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherry N, Shaikh K, McDonald C, Chowdhury Z. Stillbirth in rural Bangladesh: arsenic exposure and other etiological factors: a report from Gonoshasthaya Kendra. Bull World Health Organ. 2008;86(3):172–177. doi: 10.2471/BLT.07.043083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou WC, Chung YT, Chen HY, Wang CJ, Ying TH, Chuang CY, et al. Maternal arsenic exposure and DNA damage biomarkers, and the associations with birth outcomes in a general population from Taiwan. PLoS One. 2014;9(2):e86398. doi: 10.1371/journal.pone.0086398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quansah R, Armah FA, Essumang DK, Luginaah I, Clarke E, Marfoh K, et al. Association of arsenic with adverse pregnancy outcomes/infant mortality: a systematic review and meta-analysis. Environ Health Perspect. 2015;123(5):412–421. doi: 10.1289/ehp.1307894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman A, Vahter M, Smith AH, Nermell B, Yunus M, El Arifeen S, et al. Arsenic exposure during pregnancy and size at birth: a prospective cohort study in Bangladesh. Am J Epidemiol. 2009;169(3):304–312. doi: 10.1093/aje/kwn332. [DOI] [PubMed] [Google Scholar]
- 16.Rahman A, Persson L, Nermell B, El Arifeen S, Ekström EC, Smith AH, et al. Arsenic exposure and risk of spontaneous abortion, stillbirth, and infant mortality. Epidemiology. 2010;21(6):797–804. doi: 10.1097/EDE.0b013e3181f56a0d. [DOI] [PubMed] [Google Scholar]
- 17.Yang CY, Chang CC, Tsai SS, Chuang HY, Ho CK, Wu TN. Arsenic in drinking water and adverse pregnancy outcome in an arseniasis-endemic area in northeastern Taiwan. Environ Res. 2003;91(1):29–34. doi: 10.1016/S0013-9351(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 18.Vahter M. Mechanisms of arsenic biotransformation. Toxicology. 2002;181–182:211–217. doi: 10.1016/S0300-483X(02)00285-8. [DOI] [PubMed] [Google Scholar]
- 19.ATSDR U . Toxicological profile for arsenic. Atlanta: Agency for Toxic Substances and Disease Registry, Division of Toxicology; 2007. [PubMed] [Google Scholar]
- 20.Alauddin M, Alauddin ST, Bhattacharjee M, Sultana S, Chowdhury D, Bibi H, et al. Speciation of arsenic metabolite intermediates in human urine by ion-exchange chromatography and flow injection hydride generation atomic absorption spectrometry. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2003;38(1):115–128. doi: 10.1081/ESE-120016884. [DOI] [PubMed] [Google Scholar]
- 21.Concha G, Vogler G, Nermell B, Vahter M. Intra-individual variation in the metabolism of inorganic arsenic. Int Arch Occup Environ Health. 2002;75(8):576–580. doi: 10.1007/s00420-002-0361-1. [DOI] [PubMed] [Google Scholar]
- 22.Concha G, Nermell B, Vahter MV. Metabolism of inorganic arsenic in children with chronic high arsenic exposure in northern Argentina. Environ Health Perspect. 1998;106(6):355–359. doi: 10.1289/ehp.98106355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tam GK, Charbonneau SM, Lacroix G, Bryce F. In vitro methylation of 74As in urine, plasma and red blood cells of human and dog. Bull Environ Contam Toxicol. 1979;22(1–2):69–71. doi: 10.1007/BF02026910. [DOI] [PubMed] [Google Scholar]
- 24.Buchet JP, Lauwerys R, Roels H. Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsinate in man. Int Arch Occup Environ Health. 1981;48(1):71–79. doi: 10.1007/BF00405933. [DOI] [PubMed] [Google Scholar]
- 25.Buchet JP, Lauwerys R, Roels H. Urinary excretion of inorganic arsenic and its metabolites after repeated ingestion of sodium metaarsenite by volunteers. Int Arch Occup Environ Health. 1981;48(2):111–118. doi: 10.1007/BF00378431. [DOI] [PubMed] [Google Scholar]
- 26.Gelmann ER, Gurzau E, Gurzau A, Goessler W, Kunrath J, Yeckel CW, et al. A pilot study: the importance of inter-individual differences in inorganic arsenic metabolism for birth weight outcome. Environ Toxicol Pharmacol. 2013;36(3):1266–1275. doi: 10.1016/j.etap.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vahter M, Concha G. Role of metabolism in arsenic toxicity. Pharmacol Toxicol. 2001;89(1):1–5. doi: 10.1034/j.1600-0773.2001.d01-128.x. [DOI] [PubMed] [Google Scholar]
- 28.Jansen RJ, Argos M, Tong L, Li J, Rakibuz-Zaman M, Islam MT, et al. Determinants and consequences of arsenic metabolism efficiency among 4,794 individuals: demographics, lifestyle, genetics, and toxicity. Cancer Epidemiol Biomark Prev. 2016;25(2):381–390. doi: 10.1158/1055-9965.EPI-15-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tseng CH. A review on environmental factors regulating arsenic methylation in humans. Toxicol Appl Pharmacol. 2009;235(3):338–350. doi: 10.1016/j.taap.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Gao S, Mostofa MG, Quamruzzaman Q, Rahman M, Rahman M, Su L, et al. Gene-environment interaction and maternal arsenic methylation efficiency during pregnancy. Environ Int. 2019;125:43–50. doi: 10.1016/j.envint.2019.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hopenhayn C, Huang B, Christian J, Peralta C, Ferreccio C, Atallah R, et al. Profile of urinary arsenic metabolites during pregnancy. Environ Health Perspect. 2003;111(16):1888–1891. doi: 10.1289/ehp.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardner RM, Nermell B, Kippler M, Grandér M, Li L, Ekström EC, et al. Arsenic methylation efficiency increases during the first trimester of pregnancy independent of folate status. Reprod Toxicol. 2011;31(2):210–218. doi: 10.1016/j.reprotox.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Christian WJ, Hopenhayn C, Centeno JA, Todorov T. Distribution of urinary selenium and arsenic among pregnant women exposed to arsenic in drinking water. Environ Res. 2006;100(1):115–122. doi: 10.1016/j.envres.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Ekström EC, Goessler W, Lönnerdal B, Nermell B, Yunus M, et al. Nutritional status has marginal influence on the metabolism of inorganic arsenic in pregnant Bangladeshi women. Environ Health Perspect. 2008;116(3):315–321. doi: 10.1289/ehp.10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO Arsenic in tube well water in Bangladesh: health and economic impacts and implications for arsenic mitigation. Bull World Health Organ. 2012;90:839–846. doi: 10.2471/BLT.11.101253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodrigues EG, Bellinger DC, Valeri L, Hasan MO, Quamruzzaman Q, Golam M, et al. Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environmental Health. 2016;15(1):44. doi: 10.1186/s12940-016-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Pi-I., Bromage Sabri, Mostofa Md., Allen Joseph, Oken Emily, Kile Molly, Christiani David. Validation of a Dish-Based Semiquantitative Food Questionnaire in Rural Bangladesh. Nutrients. 2017;9(1):49. doi: 10.3390/nu9010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung J-Y, Lim H-J, Kim Y-J, Song K-H, Kim B-G, SJEh HY, et al. The separation of arsenic metabolites in urine by high performance liquid chromatographyinductively coupled plasma-mass spectrometry. 2014;29:e2014018. [DOI] [PMC free article] [PubMed]
- 39.Hsueh YM, Chiou HY, Huang YL, Wu WL, Huang CC, Yang MH, et al. Serum beta-carotene level, arsenic methylation capability, and incidence of skin cancer. Cancer Epidemiol Biomark Prev. 1997;6(8):589–596. [PubMed] [Google Scholar]
- 40.Hsueh YM, Hsu MK, Chiou HY, Yang MH, Huang CC, Chen CJ. Urinary arsenic speciation in subjects with or without restriction from seafood dietary intake. Toxicol Lett. 2002;133(1):83–91. doi: 10.1016/S0378-4274(02)00087-5. [DOI] [PubMed] [Google Scholar]
- 41.Kile Molly L., Houseman E. Andres, Breton Carrie V., Quamruzzaman Quazi, Rahman Mahmuder, Mahiuddin Golam, Christiani David C. Association between total ingested arsenic and toenail arsenic concentrations. Journal of Environmental Science and Health, Part A. 2007;42(12):1827–1834. doi: 10.1080/10934520701566819. [DOI] [PubMed] [Google Scholar]
- 42.Huyck KL, Kile ML, Mahiuddin G, Quamruzzaman Q, Rahman M, Breton CV, et al. Maternal arsenic exposure associated with low birth weight in Bangladesh. J Occup Environ Med. 2007;49(10):1097–1104. doi: 10.1097/JOM.0b013e3181566ba0. [DOI] [PubMed] [Google Scholar]
- 43.Shaheen N. Food composition tables for Bangladesh. Final Research Results. 2013;187. http://www.fao.org/fileadmin/templates/food_composition/documents/FCT_10_2_14_final_version.pdf.
- 44.Hopenhayn-Rich C, Biggs ML, Kalman DA, Moore LE, Smith AH. Arsenic methylation patterns before and after changing from high to lower concentrations of arsenic in drinking water. Environ Health Perspect. 1996;104(11):1200–1207. doi: 10.1289/ehp.961041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurttio P, Komulainen H, Hakala E, Kahelin H, Pekkanen J. Urinary excretion of arsenic species after exposure to arsenic present in drinking water. Arch Environ Contam Toxicol. 1998;34(3):297–305. doi: 10.1007/s002449900321. [DOI] [PubMed] [Google Scholar]
- 46.Styblo M, Del Razo LM, LeCluyse EL, Hamilton GA, Wang C, Cullen WR, et al. Metabolism of arsenic in primary cultures of human and rat hepatocytes. Chem Res Toxicol. 1999;12(7):560–565. doi: 10.1021/tx990050l. [DOI] [PubMed] [Google Scholar]
- 47.Karagas MR, Punshon T, Sayarath V, Jackson BP, Folt CL, Cottingham KL. Association of Rice and Rice-Product Consumption with Arsenic Exposure Early in life. JAMA Pediatr. 2016;170(6):609–616. doi: 10.1001/jamapediatrics.2016.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meharg AA, Rahman MM. Arsenic contamination of Bangladesh paddy field soils: implications for rice contribution to arsenic consumption. Environ Sci Technol. 2003;37(2):229–234. doi: 10.1021/es0259842. [DOI] [PubMed] [Google Scholar]
- 49.Taylor V, Goodale B, Raab A, Schwerdtle T, Reimer K, Conklin S, et al. Human exposure to organic arsenic species from seafood. Sci Total Environ. 2017;580:266–282. doi: 10.1016/j.scitotenv.2016.12.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gamble MV, Liu X, Ahsan H, Pilsner R, Ilievski V, Slavkovich V, et al. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect. 2005;113(12):1683–1688. doi: 10.1289/ehp.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gamble MV, Liu X, Ahsan H, Pilsner JR, Ilievski V, Slavkovich V, et al. Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid-supplementation trial in Bangladesh. Am J Clin Nutr. 2006;84(5):1093–1101. doi: 10.1093/ajcn/84.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gamble MV, Liu X, Slavkovich V, Pilsner JR, Ilievski V, Factor-Litvak P, et al. Folic acid supplementation lowers blood arsenic. Am J Clin Nutr. 2007;86(4):1202–1209. doi: 10.1093/ajcn/86.4.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters BA, Hall MN, Liu X, Parvez F, Sanchez TR, van Geen A, et al. Folic acid and Creatine as therapeutic approaches to lower blood arsenic: a randomized controlled trial. Environ Health Perspect. 2015;123(12):1294–1301. doi: 10.1289/ehp.1409396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinmaus C, Carrigan K, Kalman D, Atallah R, Yuan Y, Smith AH. Dietary intake and arsenic methylation in a U.S. population. Environ Health Perspect. 2005;113(9):1153–1159. doi: 10.1289/ehp.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoffman DJ, Sanderson CJ, LeCaptain LJ, Cromartie E, Pendleton GW. Interactive effects of arsenate, selenium, and dietary protein on survival, growth, and physiology in mallard ducklings. Arch Environ Contam Toxicol. 1992;22(1):55–62. doi: 10.1007/BF00213302. [DOI] [PubMed] [Google Scholar]
- 56.Vahter M, Marafante E. Effects of low dietary intake of methionine, choline or proteins on the biotransformation of arsenite in the rabbit. Toxicol Lett. 1987;37(1):41–46. doi: 10.1016/0378-4274(87)90165-2. [DOI] [PubMed] [Google Scholar]
- 57.Kurzius-Spencer M, da Silva V, Thomson CA, Hartz V, Hsu CH, Burgess JL, et al. Nutrients in one-carbon metabolism and urinary arsenic methylation in the National Health and Nutrition Examination Survey (NHANES) 2003-2004. Sci Total Environ. 2017;607–608:381–390. doi: 10.1016/jscitotenv201707019. [DOI] [PubMed] [Google Scholar]
- 58.Heck JE, Nieves JW, Chen Y, Parvez F, Brandt-Rauf PW, Graziano JH, et al. Dietary intake of methionine, cysteine, and protein and urinary arsenic excretion in Bangladesh. Environ Health Perspect. 2009;117(1):99–104. doi: 10.1289/ehp.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin Pi-I., Bromage Sabri, Mostofa Md., Allen Joseph, Oken Emily, Kile Molly, Christiani David. Associations between Diet and Toenail Arsenic Concentration among Pregnant Women in Bangladesh: A Prospective Study. Nutrients. 2017;9(4):420. doi: 10.3390/nu9040420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steinmaus C, Yuan Y, Kalman D, Atallah R, Smith AH. Intraindividual variability in arsenic methylation in a U.S. population. Cancer Epidemiol Biomark Prev. 2005;14(4):919–924. doi: 10.1158/1055-9965.EPI-04-0277. [DOI] [PubMed] [Google Scholar]
- 61.Kile ML, Hoffman E, Hsueh YM, Afroz S, Quamruzzaman Q, Rahman M, et al. Variability in biomarkers of arsenic exposure and metabolism in adults over time. Environ Health Perspect. 2009;117(3):455–460. doi: 10.1289/ehp.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finglas PM. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B 6, folate, vitamin B 12, pantothenic acid, biotin and choline: Elsevier; 2000. [PubMed]
- 63.Finglas Paul M. Dietary Reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin and choline. Trends in Food Science & Technology. 2000;11(8):296–297. doi: 10.1016/S0924-2244(01)00010-3. [DOI] [PubMed] [Google Scholar]
- 64.Beck KL, Houston ZL, SA MN, Kruger R. Development and evaluation of a food frequency questionnaire to assess nutrient intakes of adult women in New Zealand. Nutrition & Dietetics. 2018. [DOI] [PMC free article] [PubMed]
- 65.Heck JE, Gamble MV, Chen Y, Graziano JH, Slavkovich V, Parvez F, et al. Consumption of folate-related nutrients and metabolism of arsenic in Bangladesh. Am J Clin Nutr. 2007;85(5):1367–74. 10.1111/1747-0080.12472. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Quantiles of adjusted urinary arsenic concentrations (U-As) (μg/g creatinine). Table S2. Determinants of the proportion of arsenic metabolites in urine in mid-to-late gestation (visit 2). Figure S1. Scatter plots of DMA% and gestational weeks for all mothers. Figure S2. Correlation between adjusted U-As at visit 1 and visit 2. Figure S3. Association between unadjusted U-As and urinary creatinine at visit 1 (top) and visit 2 (bottom).
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to institutional review board specifications (but are available from the corresponding author on reasonable request).