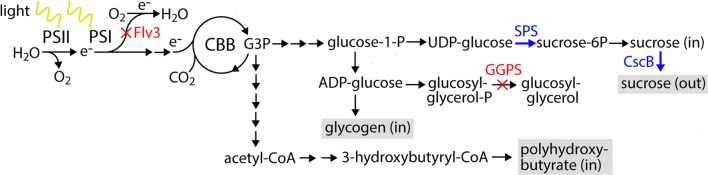
Fig. 1.
Simplified representation of pathways engineered in Synechocystis to evaluate the possibility to enhance the photosynthetic electron flux to target products by the inactivation of the flavodiiron protein 3 (Flv3) and improving the strength of the electron sink (see Table 1 for strain descriptions). The engineering strategy made use of the native capacity of cyanobacterial cells to alleviate osmotic stress by the production of intracellular sucrose and glucosylglycerol as osmoprotective agents. Specific genetic modifications introduced in Synechocystis to maximize the production of sucrose include (i) the inactivation (red) of Flavodiiron protein 3 (Flv3 encoded by sll0550) which is involved in a photoprotective Mehler-like reaction of the photosynthetic electron transfer chain i.e. the loss of excited electrons to molecular oxygen, and (ii) the overexpression (blue) of sucrose permease (CscB from E. coli) responsible for the active transport of sucrose out from the cell into the culture medium. In addition, the modifications include (iii) the inactivation (red) of glucosylglycerolphosphate synthase (ggpS encoded by sll1566) responsible for a committed step in the biosynthesis of glucosylglycerol, competing with the sucrose biosynthesis pathway, and (iv) the overexpression (blue) of sucrose phosphate synthase (SPS encoded by sll0045) which enhances one of the potentially limiting steps, conversion of UDP-glucose to sucrose-6-phosphate. The target product sucrose and the storage compounds, polyhydroxybutyrate and glycogen, quantitated in the study are shown in grey background. (See Additional file 1: Table S2 for a more comprehensive list of the enzymatic reactions in the pathways, and the production and use of ATP and NADPH)