Short abstract
Introduction
Hepatocellular carcinoma (HCC) is a common gastrointestinal cancer that occurs worldwide, and conventional transarterial chemoembolization (cTACE) is one of the first treatment choices for advanced HCC. However, biochemical factors and comorbidity have seldom been reported in the long-term outcomes.
Methods
This retrospective cohort study included 444 HCC patients who underwent cTACE-based therapy in 2010 to 2012. Survival outcomes were analyzed using a Kaplan–Meier curve and Cox regression analysis.
Results
The mean age was 62.1 ± 12.5 years, and 74.3% were men. Analysis of the mean biochemical values indicated that the presence of portal vein thrombosis, α-fetoprotein (AFP) >200 ng/mL, AJCC 7th stage III, diabetes, albumin <3 g/dL, and hemoglobin were significantly and independently associated with poorer long-term outcomes.
Discussion
The presence of venous thrombus and elevation of AFP levels are the most important factors in cTACE treatment. The host factors, including metabolic status and liver damage, should be evaluated in these patients.
Keywords: Hepatocellular carcinoma, TACE, diabetes, portal vein thrombosis, α-fetoprotein, albumin, hemoglobin
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cancer globally.1,2 Curative therapies for HCC such as surgical resection, radiofrequency ablation, and liver transplantation offer a good survival benefit. However, most HCC patients with multiple lesions or vascular invasion did not qualify for the curative treatment.3,4 Since its first introduction four decades ago, transarterial chemoembolization (TACE) has been shown to be a valuable therapy for HCC patients with the intermediate-stage (B) of the Barcelona Clinic Liver Cancer (BCLC) staging system.5,6 Over the years, variable technical aspects have been proposed including different embolic agents, drug-eluting beads (DEB TACE), treatment interval and combination therapy with targeted therapy or radiotherapy (transarterial radioembolization). Conventional TACE (cTACE), which involves injecting a mixture of cytotoxic agents and ethiodol oil using interventional radiological technique, was the mainstay of popular TACE treatment method during the three to four decades of its development, especially in Japan and Taiwan.7–9
The survival benefit of cTACE has been shown in BCLC stage B HCC patients in randomized trials and in meta-analyses.10–12 Through the 1990s, selective and superselective approach TACE were developed to control the damage to normal parenchyma and improve the treatment response.13–15 The 4th International Kyoto Liver Cancer Symposium (IKLS) declared that the tumor size, number, location, and grading were the most concerning host factors for cTACE, and treatment failure was discussed to improve the outcomes.16 However, few studies considered host factors such as comorbidities (e.g. diabetes), transaminase levels, and nutritional status. Recently, one study on early mortality after partial hepatectomy reported that surgical complications, low albumin levels, diabetes, and high α-fetoprotein (AFP) levels were independent predictive factors.17 Therefore, the treatment modality should be carefully selected because the liver function and host conditions could possibly affect the oncologic outcomes.
This retrospective study aimed to analyze the influence biochemical factors and diabetes of HCC patients who underwent cTACE as a primary treatment at a northern Taiwan tertiary center.
Materials and methods
Patients and study design
Between January 2010 and December 2012, 444 HCC patients who received the primary treatment of cTACE at a northern Taiwan tertiary center were included in this study. The clinical host factors and HCC risk factors such as demographic parameters, clinical parameters, hepatitis markers, tumor-related parameters, liver functional reserve, treatment modalities, and outcomes were recorded and analyzed. This retrospective study was approved by the Institutional Review Board of Chang Gung Medical Foundation (201701003B0); written informed consent was not required because of the retrospective nature of the study, and all identifying information was protected for the enrolled patients and their families. The treatment protocol for HCC was based on the BCLC staging system. Patients were considered to exhibit unresectable HCC when at least one of the following conditions was present: multiple nodules, liver cirrhosis, inadequate liver function reserve for hepatectomy, and/or comorbidities with contraindications for general anesthesia. Patients who were not candidates for surgery or ablation therapy were potentially eligible for cTACE when the maximum tumor diameter was larger than 5 cm or if the tumor was close to major vascular or biliary structures.
Diagnosis and management
The treatment plans were discussed by the multidisciplinary panel that included hepatologists, hepatobiliary surgeons, oncologists, and interventional radiologist to propose the optimal treatment option for each individual patient. cTACE-based procedures were performed every 3 to 4 months by interventional radiologists. Serial TACE procedures were withheld when there was liver decompensation or extrahepatic spread. For patients who exhibited extrahepatic spread during serial TACE sessions, treatment options including targeted therapy, radiotherapy/chemotherapy, and the best supportive care with or without concurrent cTACE were determined again by the multidisciplinary panel. For patients who developed hepatic tumor recurrence after hepatectomy (hepatectomy and recurrence group) but were not eligible for resection or radiofrequency ablation, team discussion and serial cTACE therapy was a common strategy.
cTACE procedures
Cross-section abdominal imaging including contrast-enhanced abdominal computed tomography (CT) and magnetic resonance imaging (MRI) with dedicated dynamic liver protocol were performed before cTACE sessions in all patients to plan the procedure. cTACE procedures were performed by dedicated interventional radiologists in accordance with the standard protocols. Pre-procedure hydration and prophylactic antibiotics were administered. Post-cTACE, all patients were admitted for in-hospital monitoring for at least 24 hours.
Vascular access was established via a common femoral artery puncture using the Seldinger method. A 4-French catheter (Radifocus Optitorque, Terumo Corporation, Tokyo, Japan) was used to cannulate the visceral arteries (celiac trunk, common hepatic artery and its branches, and superior mesenteric artery). Digital subtraction angiography was performed to evaluate the arterial vasculature and the tumor vessels, as well as the portal vein patency. Superselective cannulation with the tip of the catheter further advancing into the branches of the right or left hepatic artery was performed with a 2.7-French coaxial microcatheter system (Progreat microcatheter, Terumo Corporation). Chemoembolization was performed with a mixture of doxorubicin hydrochloride (75 mg/m2 body surface area) with 10 mL of lipiodol (Lipiodol Ultra-fluide, Guerbet, Aulnay-sous-Bois, France). Gelatin sponge pledges were then injected to ensure stagnation of the blood flow in the embolized vessel. A total of 2205 cTACE sessions (mean, 3.5 sessions per patient; maximum, 27 sessions) were performed; 16 (3.6%) and 271 (71.0%) of patients underwent hepatectomies and ablation therapy in the subsequent treatment sessions.
Follow-up and surveillance for HCC
The follow-up protocol included outpatient visits and monitoring of serum liver biochemistry with imaging examinations (abdominal ultrasonography, contrast-enhanced CT, or MRI) every 3 months. Tumor progression, remission, or recurrence was determined and recorded.
Statistical analysis
Descriptive data including the mean, standard deviation, median, interquartile range (IQR), frequencies, and percentages were used to characterize the patients. Each patient’s last visit or death was recorded for the survival analysis. Categorical data were analyzed using the chi-squared test or Fisher’s exact test, while continuous variables were analyzed using the Student’s t-test. Survival rates in each group were determined by the log-rank test and Kaplan–Meier survival curves. Cox regression analysis was used to identify the prognostic factors that were associated with overall survival. All statistical analyses were performed using SPSS version 19 (IBM Corp., Armonk, NY, USA). P-values less than 0.05 were considered to be statistically significant.
Results
Patient demographics
The mean age was 62.1 ± 12.5 years. There were 330 (74.3%) males and 114 (25.7%) females. Overall, 240 (54.1%) patients had hepatitis B and 188 (42.3%) had hepatitis C. The Child–Pugh classification indicated that 384 (86.5%) patients were in class A, while the remaining 60 (13.5%) were in class B or C. Based on the American Joint Committee on Cancer (AJCC) TNM staging, 7th edition,18 at the time of diagnosis or recurrence, 322 patients were in stage I/II and 122 were in stage III (Table 2). Among the patients, 355 (80.0%) underwent serial cTACE as the primary treatment while 89 (20.0%) belonged to the hepatectomy and recurrence group. The mean follow-up time was 31.8 months (IQR, 13.5–49.2 months). The clinical and demographic data are summarized in Table 1.
Table 2.
Tumor characteristics in the cTACE cohort.
| Tumor | |
| Single | 134 (30.2) |
| Multiple | 310 (69.8) |
| Lobe | |
| Right | 141 (31.8) |
| Left | 44 (9.9) |
| Bilobar | 152 (34.2) |
| Central | 107 (24.1) |
| Venous thrombus | |
| Yes | 58 (13.1) |
| AJCC 7th Stage | |
| I/II | 322 (72.5) |
| III | 122 (27.5) |
| TACE approach | |
| Superselective | 356 (80.2) |
| Lobar | 88 (19.8) |
| Treatment | |
| Primary | 355 (80.0) |
| Recurrence | 89 (20.0) |
Values indicate numbers (percentages) of patients with each characteristic.
cTACE, conventional transarterial chemoembolization; TACE, transarterial chemoembolization; AJCC, American Joint Committee on Cancer.
Table 1.
cTACE cohort demographic data.
| Age (years) | 62.1 ± 12.5 (53–72) |
| Sex (Male/Female), n (%) | 330 (74.3%)/114 (25.7%) |
| BMI (kg/m2) | 25.1 ± 4.3 (22.6–27.1) |
| Comorbidity | 225 (50.7%) |
| Diabetes | 117 (26.4%) |
| ESRD | 15 (3.4%) |
| Hb (g/dL) | 12.5 ± 2.1 (11.0–13.9) |
| Platelet (×1000/µL) | 145.7 ± 89.4 (81.0–188.7) |
| Total Bilirubin (mg/dL) | 0.9 ± 0.6 (0.5–1.1) |
| AST (U/L) | 65.0 ± 51.3 (36.0–83.0) |
| ALT (U/L) | 53.1 ± 44.9 (26.0–63.0) |
| Alk-p (U/L) | 114.7 ± 64.1 (74.0–137.0) |
| Albumin (g/dL) | 3.7 ± 0.6 (3.4–4.1) |
| Creatinine (mg/dL) | 1.0 ± 1.2 (0.6–1.0) |
| AFP (ng/mL) | 10734.1 ± 67855.4 (8.6–568.1) |
| Cirrhosis | 284 (64.0) |
| HBV | 240 (54.1) |
| HCV | 188 (42.3) |
| Child A | 384 (86.5) |
| Child B/C | 60 (13.5) |
| Follow-up (months) | 31.8 ± 20.8 (13.5–49.2) |
Values in parentheses: percentage or 25–75 percentile.
cTACE, conventional transarterial chemoembolization; BMI, body mass index; ESRD, end stage renal disease; Hb, hemoglobin; AST, aspartate aminotransferase; ALT, alanine aminotransferase (ALT); Alk-p, alkaline phosphatase; AFP, α-fetoprotein; HBV, hepatitis B virus; HCV, hepatitis C virus; cTACE, conventional transarterial chemoembolization.
Tumor parameters and response
The detailed characteristics of the tumors are shown in Table 2. Multifocal tumors were observed in most patients, and most patients exhibited TNM stage I/II tumors. Most patients underwent serial cTACE as the primary treatment.
Survival outcome
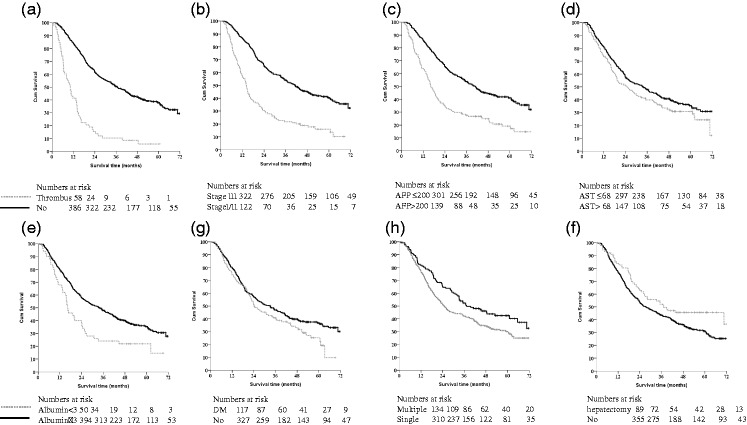
During the follow-up period, 286 (64.4%) patients died; the cause of death was HCC and liver decompensation in 281 (98.2%). The overall 1-, 3-, and 5-year survival rates were 78.7%, 45.3%, and 33.8%, respectively. To analyze the significant prognostic factors, log-rank tests were used to identify risk factors for overall survival; these are summarized in Table 3. Kaplan–Meier survival analysis showed significant and marginally significant differences in the selected factors in 444 HCC patients: venous thrombus (p<0.001), AJCC 7th stage III (p<0.001), AFP >200 ng/mL (p<0.001), bilobar lesions (p<0.048), multiple lesions (p<0.01), selective approach (p<0.005), albumin ≤3.0 g/dL (p<0.001), AST over twice the normal limit (>68 U/L), diabetes, hemoglobin (<8.0 g/dL; p<0.019), and recurrence after hepatectomy (p<0.032). Figure 1 showed the representative variables. A multivariate Cox regression analysis (Table 3) indicated that the presence of portal venous thrombosis (hazard ratio [HR]: 2.692 [95% confidence interval (CI) 1.771–4.091] p<0.001), AFP >200 ng/mL (HR: 1.726 [1.341–2.221], p<0.001), AJCC 7th stage III (HR: 1.435 [1.023–2.012], p = 0.036), diabetes (HR: 1.371 [1.053–1.784], p = 0.019), albumin <3 g/dL (HR: 1.726 [1.216–2.451], p = 0.002), and hemoglobin (<8.0 g/dL, HR: 2.258 [1.123–4.538], p = 0.022), were significantly and independently associated with poorer long-term outcomes.
Table 3.
Overall survival in the HCC cTACE cohort.
|
Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Factors | HR | 95% CI | P value | HR | 95% CI | P value |
| Age (years) | ||||||
| >70 vs. ≤70 | 0.947 | 0.732–1.227 | 0.681 | |||
| Sex | ||||||
| Female vs. Male | 0.864 | 0.661–1.130 | 0.285 | |||
| Comorbidity | ||||||
| Yes vs. No | 0.931 | 0.739–1.174 | 0.547 | |||
| Diabetes | ||||||
| Yes vs. No | 1.279 | 0.991–1.651 | 0.058 | 1.371 | 1.053–1.784 | 0.019 |
| Hepatectomy and recurrence | ||||||
| Yes vs. No | 0.711 | 0.520–0.972 | 0.032 | 0.899 | 0.650–1.242 | 0.518 |
| Cirrhosis | ||||||
| Yes vs. No | 1.093 | 0.856–1.396 | 0.476 | |||
| Multiple | ||||||
| Yes vs. No | 1.411 | 10.85–1.836 | 0.010 | 1.319 | 0.968–1.798 | 0.080 |
| Venous thrombus | ||||||
| Yes vs. No | 3.919 | 2.893–5.310 | <0.001 | 2.692 | 1.771–4.091 | <0.001 |
| Bilobar | ||||||
| Yes vs. No | 1.274 | 1.002–1.620 | 0.048 | 1.136 | 0.857–1.505 | 0.376 |
| AJCC 7th Stage | ||||||
| ≥3 vs. 1,2 | 2.559 | 2.002–3.271 | <0.001 | 1.435 | 1.023–2.012 | 0.036 |
| Selective approach | ||||||
| No vs. Yes | 1.485 | 1.126–1.959 | 0.005 | 1.154 | 0.859–1.551 | 0.342 |
| AFP 200 ng/mL | ||||||
| Higher vs. Lower | 2.105 | 1.655–2.678 | <0.001 | 1.726 | 1.341–2.221 | <0.001 |
| Bilirubin 1.3 mg/dL | ||||||
| Higher vs. Lower | 1.213 | 0.876–1.678 | 0.243 | |||
| AST 68 U/L | ||||||
| Higher vs. Lower | 1.257 | 0.988–1.601 | 0.062 | 1.257 | 0.978–1.616 | 0.074 |
| ALT 72 U/L | ||||||
| Higher vs. Lower | 0.918 | 0.688–1.225 | 0.562 | |||
| Albumin 3.0 g/dL | ||||||
| Lower vs. Higher | 1.731 | 1.238–2.420 | 0.001 | 1.726 | 1.216–2.451 | 0.002 |
| Hemoglobin 8.0 g/dL | ||||||
| Lower vs. Higher | 2.169 | 1.115–4.221 | 0.019 | 2.258 | 1.123–4.538 | 0.022 |
HCC, hepatocellular carcinoma; cTACE, conventional transarterial chemoembolization; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, α-fetoprotein; AJCC, American Joint Committee on Cancer; CI, confidence interval.
Figure 1.
Kaplan–Meier survival analysis for HCC patients with cTACE based treatment.
Kaplan–Meier survival analysis showing the differences in the selected factors in 444 HCC patients (gray dotted line): (a) venous thrombus (p<0.001); (b) AJCC 7th stage III (p<0.001); (c) AFP >200 ng/mL (p<0.001); (d) AST over twice normal limit (>68 U/L); (e) albumin ≤3.0 g/dL (p<0.001); (f) diabetes; (g) multiple (p<0.010); and (h) recurrence after hepatectomy (p<0.032).
HCC, hepatocellular carcinoma; cTACE, conventional transarterial chemoembolization; AFP, α-fetoprotein; AST, aspartate aminotransferase.
Discussion
Currently, cTACE is the treatment of choice for intermediate-stage and advanced BCLC-stage HCC; contraindications included extrahepatic spreading and liver decompensation. In this study, patients with portal vein thrombus, AJCC 7th stage III HCC and high AFP levels exhibited poor outcomes. Moreover, we found that elevated transaminase level, poor nutrition status (specifically in terms of albumin), and the presence of diabetes were independent significant prognostic factors. Llovet et al. reported that the 1- and 2-year survival rates of HCC patients were 82% and 63%, respectively.19 Since 2000, lipiodol-based anticancer drug delivery and a superselective approach have been extensively developed, with significant improvements in treatment efficacy. Takayasu et al. reported 1-, 3-, and 5-year survival rates of 82%, 47%, and 26%, respectively;15 in the current study, these rates were 78.7%, 45.3%, and 33.8%, respectively. Additionally, biochemical data, information regarding diabetes and other comorbidities, and nutritional statuses were collected to assess host conditions. The results showed that careful patient selection and improved patient care quality are necessary in the future.
Patients with TNM stage III HCC had poorer outcomes than patients with stage I or II; this observation was in accordance with that of a previous study group.15 Tumor size measurements were inconsistent in our study because some tumors exhibited an infiltrative phenotype, and there may have been bias among the estimations by different radiologists; thus, these data were not analyzed in our study. Multiple and bilobar lesions are common indications for cTACE; however, they were not independent factors in Cox regression analysis, thus indicating that cTACE provided acceptable treatment efficacy. However, portal venous thrombosis was associated with very poor outcomes; therefore, treatment with cTACE should be cautiously considered for such patients. The meta-analysis showed that median survival (95% CI) time was 8 (range, 5–15) months and 1-, 3-, and 5-year survival rates were 29%, 4%, and 1%, respectively.20
Liver parenchyma damage is an independent factor for HCC after partial hepatectomy for HCC and antiviral treatment is an effective treatment strategy for better outcomes.21,22 Liver damage was reported as an independent factor in the cTACE cohort analyzed by Takayasu et al.; cirrhosis and Child–Pugh status represent the liver functional reserve and could be associated with liver damage after cTACE.15,23 Higher AFP and AST levels may be responsible for the poor outcomes in this study: higher AFP levels suggest micro-metastases, which were not detected, whereas higher AST levels may be related to chronic viral infection and liver damage. The alkaline phosphatase level was a prognostic factor in univariate analysis, but the increase in AST showed a stronger association. Both were predictors of liver functional test results in HCC outcomes.21 Additionally, antiviral therapy could be an effective adjuvant combined therapy for TACE both in hepatitis B or C-infected patients and those who undergo surgery or ablation therapy.24,25
Post-hepatectomy HCC recurrence is also a common indication for cTACE because of multiple lesions and the risk of declining liver function.26 Patients typically attended regular follow-up and were treated immediately when recurrence was observed. Additionally, postoperative recurrence often showed a less invasive tumor pattern, more multiple lesions, and reduced incidence of venous thrombus. Regular surveillance with serum tests, sonography, and dynamic studies may assist in accurate diagnosis and appropriate treatment planning.
Many studies have reported diabetes as a factor that is associated with hepatocarcinogenesis and recurrence. A large meta-analysis involving approximately 3 million people from 13 case-control studies determined that diabetes was associated with the risk of incident HCC;27 another meta-analysis of 17 case-control studies and 32 cohort studies concluded that type 2 diabetes increased HCC prevalence and mortality.28 Similarly, Dyal et al. reported that diabetes was associated with an increased risk of HCC.29 Notably, diabetes was reported to be associated with hepatocarcinogenesis, and metformin was reported to decrease the risk.30–33 Diabetes and low albumin levels were reported to be independent predictors of postoperative mortality and early recurrence after partial hepatectomy.17 Metabolomic studies have reported that derangements of metabolism in HCC patients resulted in higher glucose, propylene glycol, and ethanol levels, compared with the control cohort.34 Diabetes and nutrition are both host metabolic factors that are associated with poor prognoses in HCC patients. However, we did not closely analyze metformin in this study because of our small cohort size, as well as unclear drug dosage and maintenance intervals.
Conclusions
This study showed that advanced AJCC stage, venous thrombus, and AFP levels were the most significant prognostic factors in cTACE treatment; however, this study identified that biochemical factors and diabetes were important in HCC patient outcomes with cTACE treatment. Further work focused on the association between diabetes, biochemical factors, and clinical outcome could help to identify the influence of host factors.
List of abbreviations
HCC, hepatocellular carcinoma; cTACE, conventional transarterial chemoembolization; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, α-fetoprotein; HR, hazard ratio; CI, confidence interval; BCLC, Barcelona Clinic Liver Cancer; TACE, transarterial chemoembolization; IKLS, International Kyoto Liver Cancer Symposium; CT, computed tomography; MRI, magnetic resonance imaging; IQR, interquartile range; AJCC, American Joint Committee on Cancer
Acknowledgements
We are grateful to Ms. Yi-Ping Liu for the data collection and the technological support of the Department of Cancer Center, Chang Gung Memorial Hospital, Linkou branch. The statistics study was supported by Prof. Yun-Shien Lee, Department of Biotechnology, Ming-Chuan University, Taoyuan 33348, Taiwan.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The study was supported by Chang Gung Medical Foundation CORPG 1G00071 (CORPG3G0681) and CMRPG 3D0511-3. The authors declare that there is no conflict of interest.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. DOI: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization: Cancer: Fact Sheet. http://www.who.int/mediacentre/factsheets/fs297/en/ (2017).
- 3.Saraswat VA, Pandey G, Shetty S. Treatment algorithms for managing hepatocellular carcinoma. J Clin Exp Hepatol 2014; 4: S80–S89. DOI: 10.1016/j.jceh.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908–943. DOI: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015; 35: 2155–2166. DOI: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999; 19: 329–338. DOI: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 7.Ho SY, Liu PH, Hsu CY, et al. Prognostic role of noninvasive liver reserve markers in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. PLoS One 2017; 12: e0180408. DOI: 10.1371/journal.pone.0180408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arii S, Yamaoka Y, Futagawa S, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology 2000; 32: 1224–1229. DOI: 10.1053/jhep.2000.20456. [DOI] [PubMed] [Google Scholar]
- 9.Lee TY, Lin CC, Chen CY, et al. Combination of transcatheter arterial chemoembolization and interrupted dosing sorafenib improves patient survival in early-intermediate stage hepatocellular carcinoma: a post hoc analysis of the START trial. Medicine 2017; 96: e7655. DOI: 10.1097/md.0000000000007655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horikawa M, Miyayama S, Irie T, et al. Development of conventional transarterial chemoembolization for hepatocellular carcinomas in Japan: historical, strategic, and technical review. AJR Am J Roentgenol 2015; 205: 764–773. DOI: 10.2214/ajr.15.14825. [DOI] [PubMed] [Google Scholar]
- 11.Tsurusaki M, Murakami T. Surgical and locoregional therapy of HCC: TACE. Liver Cancer 2015; 4: 165–175. DOI: 10.1159/000367739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji SK, Cho YK, Ahn YS, et al. Multivariate analysis of the predictors of survival for patients with hepatocellular carcinoma undergoing transarterial chemoembolization: focusing on superselective chemoembolization. Korean J Radiol 2008; 9: 534–540. DOI: 10.3348/kjr.2008.9.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyayama S, Matsui O, Yamashiro M, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol 2007; 18: 365–376. DOI: 10.1016/j.jvir.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002; 35: 1164–1171. DOI: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 15.Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 2006; 131: 461–469. DOI: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 2014; 87: 22–31. DOI: 10.1159/000368142. [DOI] [PubMed] [Google Scholar]
- 17.Hsu HY, Yu MC, Lee CW, et al. RAM score is an effective predictor for early mortality and recurrence after hepatectomy for hepatocellular carcinoma. BMC Cancer 2017; 17: 742. DOI: 10.1186/s12885-017-3748-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng CH, Lee CF, Wu TH, et al. Evaluation of the new AJCC staging system for resectable hepatocellular carcinoma. World J Surg Oncol 2011; 9: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002; 359: 1734–1739. DOI: 10.1016/s0140-6736(02)08649-x. [DOI] [PubMed] [Google Scholar]
- 20.Silva JP, Berger NG, Tsai S, et al. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis. HPB (Oxford) 2017; 19: 659–666. DOI: 10.1016/j.hpb.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Yu MC, Chan KM, Lee CF, et al. Alkaline phosphatase: does it have a role in predicting hepatocellular carcinoma recurrence? J Gastrointest Surg 2011; 15: 1440–1449. DOI: 10.1007/s11605-011-1537-3. [DOI] [PubMed] [Google Scholar]
- 22.Wei X, Li N, Li S, et al. Hepatitis B virus infection and active replication promote the formation of vascular invasion in hepatocellular carcinoma. BMC Cancer 2017; 17: 304. DOI: 10.1186/s12885-017-3293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waked I, Berhane S, Toyoda H, et al. Transarterial chemo-embolisation of hepatocellular carcinoma: impact of liver function and vascular invasion. Br J Cancer 2017; 116: 448–454. DOI: 10.1038/bjc.2016.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu SL, Zhong JH, Ke Y, et al. Comparative efficacy of postoperative transarterial chemoembolization with or without antiviral therapy for hepatitis B virus-related hepatocellular carcinoma. Tumour Biol 2015; 36: 6277–6284. DOI: 10.1007/s13277-015-3313-6. [DOI] [PubMed] [Google Scholar]
- 25.Manthravadi S, Paleti S, Pandya P. Impact of sustained viral response postcurative therapy of hepatitis C-related hepatocellular carcinoma: a systematic review and meta-analysis. Int J Cancer 2017; 140: 1042–1049. DOI: 10.1002/ijc.30521. [DOI] [PubMed] [Google Scholar]
- 26.Takayasu K. Transarterial chemoembolization for hepatocellular carcinoma over three decades: current progress and perspective. Jpn J Clin Oncol 2012; 42: 247–255. DOI: 10.1093/jjco/hys020. [DOI] [PubMed] [Google Scholar]
- 27.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006; 4: 369–380. DOI: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Kang D, Cao W, et al. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev 2012; 28: 109–122. DOI: 10.1002/dmrr.1291. [DOI] [PubMed] [Google Scholar]
- 29.Dyal HK, Aguilar M, Bartos G, et al. Diabetes mellitus increases risk of hepatocellular carcinoma in chronic hepatitis C virus patients: a systematic review. Dig Dis Sci 2016; 61: 636–645. DOI: 10.1007/s10620-015-3983-3. [DOI] [PubMed] [Google Scholar]
- 30.Donadon V, Balbi M, Mas MD, et al. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int 2010; 30: 750–758. DOI: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 31.Lai SW, Chen PC, Liao KF, et al. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol 2012; 107: 46–52. DOI: 10.1038/ajg.2011.384. [DOI] [PubMed] [Google Scholar]
- 32.Singh S, Singh PP, Singh AG, et al. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol 2013; 108: 881–891; quiz 892. DOI: 10.1038/ajg.2013.5. [DOI] [PubMed] [Google Scholar]
- 33.Zhou YY, Zhu GQ, Liu T, et al. Systematic review with network meta-analysis: antidiabetic medication and risk of hepatocellular carcinoma. Sci Rep 2016; 6: 33743. DOI: 10.1038/srep33743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fages A, Duarte-Salles T, Stepien M, et al. Metabolomic profiles of hepatocellular carcinoma in a European prospective cohort. BMC Med 2015; 13: 242. DOI: 10.1186/s12916-015-0462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]