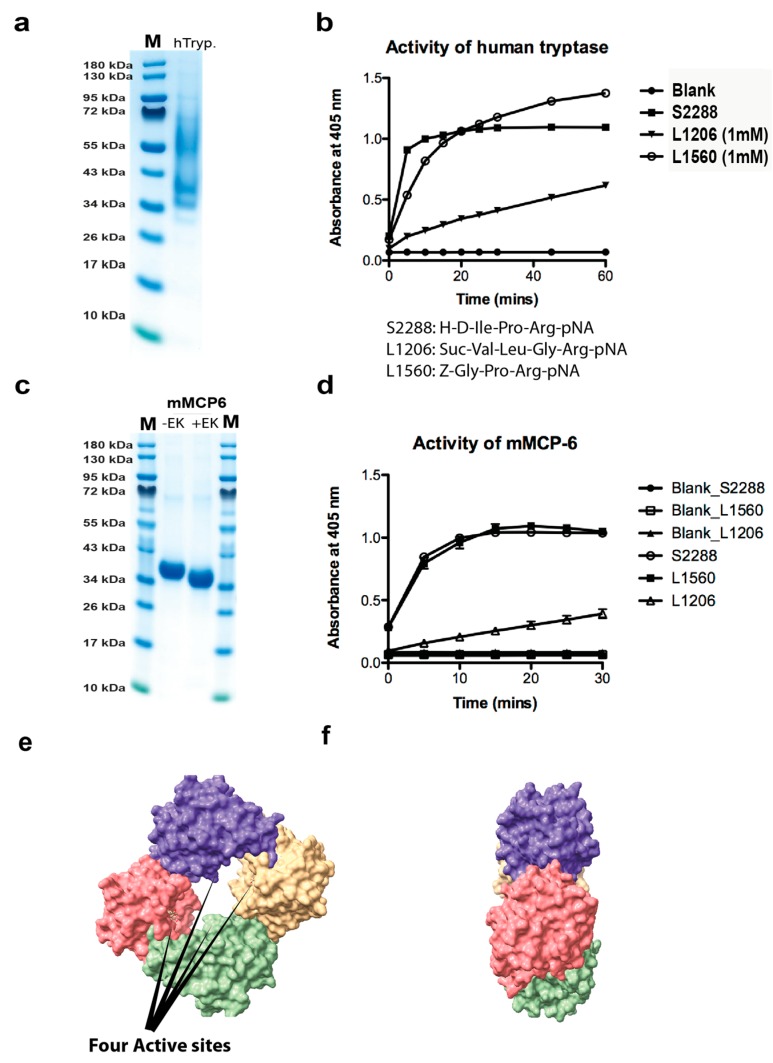
Figure 1.
Analysis of the recombinant human and mouse mast cell tryptases used for the substrate analysis by SDS-PAGE and chromogenic substrate assay. (a) Approximately two micrograms of the recombinant human tryptase was separated on a 4–12% gradient SDS-PAGE gel and stained with colloidal Coomassie blue solution. Several bands starting at approximately 36 kDa up to almost 70 kDa were seen, indicating heterogenous glycosylation. (b) The activity of the human recombinant tryptase was assayed against three different chromogenic substrates. All three are substrates for tryptic enzymes due to the Arg in the P1 position. The human tryptase showed good activity against all three, but the best activity against the two substrates occurred when the Arg was preceded by a Pro residue. (c) The purified recombinant mouse mast cell tryptase (mMCP-6) before and after enterokinase cleavage. (d) The activity of the mouse recombinant tryptase was assayed against three different chromogenic substrates. The substrate preference for the mouse enzyme was almost identical to its human counterpart shown in (b). (e,f) Three-dimensional structural models of the human tryptase tetramer (PDB No: 2fs9). The space filling model of the human MC tryptase is shown from two angles, from the front looking into the tetramer and from the side [20]. The four active sites in the middle of the tetramer are marked by four arrows. The pictures were visualized in the UCSF Chimera program.