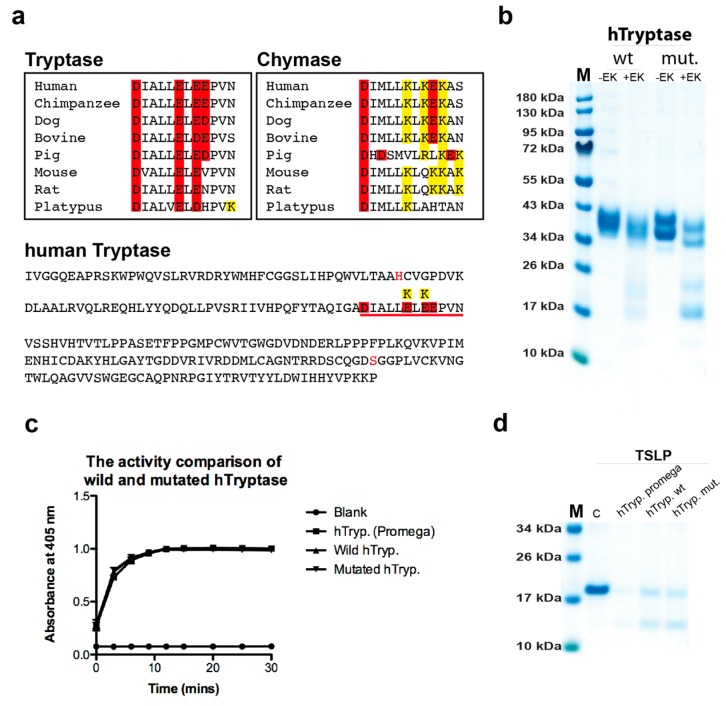
Figure 6.
The effect on cleavage by mutating two negatively charged residues close to the active site of human tryptase. By analysis of the primary structure of the human tryptase we have observed a marked difference concerning charge close to the active site between tryptases and chymases. C-terminally of the asparagine residue of the catalytic triad all tryptases have a negative patch of three negatively charged residues (a). This region including the Asp of the catalytic triad and the three negatively charged residues is underlined in red in the sequence of the entire human tryptase shown in the bottom panel of (a). In this region, all chymases instead have two positively charged residues and one negatively charged amino acid (a). Could this region be involved in target selection? Two residues that differ between tryptases and chymases in the human tryptase was therefore mutated to study their involvement on cleavage (a). The wt and mutant enzymes were produced in HK-293-EBNA cells and activated by enterokinase cleavage, lowering the pH to 6.0, and adding heparin. (b) SDS-PAGE gel analysis of wt and mutant enzyme before and after enterokinase cleavage. (c) Cleavage of chromogenic substrates and (d) the cleavage of human TSLP by the pichia produced enzyme and the wt and mutated tryptase produced in mammalian cells.