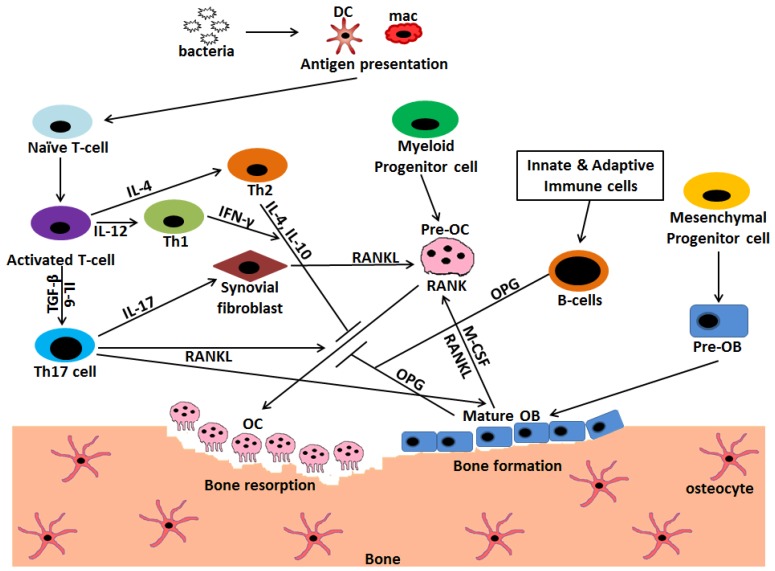
Figure 3.
Interaction and crosstalk between immune cells, osteoblasts, and osteoclasts mediated via different cytokines regulate the extent of bone erosion during infection. Osteoclasts (OC) are the cells of myeloid origin that degrade the bone matrix, whereas osteoblasts (OB) are the bone forming cells that have mesenchymal origin. Bacterial entry into the host initiates a complex crosstalk between immune cells, mainly, T and B cells with osteoclasts. The invading pathogen is phagocytized and presented by macrophages (mac) and dendritic cells (DC) to activate T cells. These activated T cells further get differentiated into T helper (Th) 1, Th2, and Th17 subsets. Th17 is the prominent osteoclastogenic T cell subset which expresses receptor activator of NF-κB ligand (RANKL) and induces the formation of osteoclasts by binding to RANK on pre-osteoclasts. It also secretes IL-17 that induces the synovial fibroblasts as well as osteoblasts to express RANKL further leading to osteoclastogenesis. Contrarily, Th1 and Th2 subset of T cells inhibits osteoclastogenesis by secreting cytokines INF-γ, IL-4 and IL-10 respectively. B cells, being activated by innate and adaptive immune cells, secrete OPG, which acts as an inhibitor to osteoclast formation process. Osteoblasts also secrete macrophage-colony-stimulating factor (M-CSF) and RANKL that aids in the process of osteoclastogenesis.  Stimulation;
Stimulation;  Inhibition.
Inhibition.