Abstract
Introduction
There is currently a lack of translatable, preclinical models of low back pain (LBP). Chymopapain, a proteolytic enzyme used to treat lumbar intervertebral disc (IVD) herniation, could induce discogenic LBP. The current study developed a behavioral model of discogenic LBP in nonhuman primates. Significant brain activation is observed in clinical LBP. Thus, the current study also sought to define brain activation over time in a macaque with discogenic LBP.
Methods
Responses to pressure applied to the back at L4/L5 were measured in eight adult male Macaca fasciculata using a pressure algometer. The nucleus pulpous of the IVD between L4 and L5 was aspirated and chymopapain (1 mg/mL) was injected under fluoroscopic guidance (n = 2). In two macaques, the nucleus pulpous was only aspirated. Brain activation in response to pressure applied to the lower back was assessed using a 3.0T magnetic resonance imaging scanner in four macaques before and 1, 3, 9, and 14 days after treatment.
Results
The mean (±SD) response pressure before treatment was 1.4 ± 0.1 kg. One day after chymopapain treatment, the response pressure decreased to 0.6 ± 0.05 kg (P < 0.01), suggestive of pressure hypersensitivity. Over time, the pressure thresholds following chymopapain treatment gradually returned to normal. Following aspiration only, the response pressure was 1.4 ± 0.05 kg, which was not significantly different from the uninjured controls. There was activation of the secondary somatosensory cortex and insular cortex one and three days after chymopapain treatment; there was no activation following aspiration only.
Conclusions
Enzymatic treatment of the nucleus pulpous leads to acute LBP and pressure-evoked activation in pain-related brain areas. The current model of discogenic LBP parallels clinical LBP and could be used to further elaborate the mechanism of acute LBP.
Keywords: chymopapain, discogenic low back pain, nonhuman primate, pressure test, brain activation, functional magnetic resonance imaging, secondary somatosensory cortex, insular cortex
Introduction
Low back pain (LBP) is a common clinical problem that leads to significant disability, which may ultimately result in adverse socioeconomic impact1,2). Pain is inherently subjective, and visual analog scales are used to quantify pain intensity. However, to better understand the mechanism and standardize the measurement of treatment efficacy, objective LBP measures are needed. There are a number of therapeutic interventions, including pharmacological, psychological, and behavioral, that may be useful in treating LBP patients3). However, it is currently not known which of these treatments has the greatest efficacy since currently used outcome measures are subjective.
Neuroimaging has demonstrated morphological and functional changes in the brains of chronic pain patients4-6). Brain activation, as observed with functional magnetic resonance imaging (fMRI), reflects activation of large ensembles of neurons and local field potentials in response to stimulation. While pain itself is a subjective experience, brain activity could be useful as an objective marker to reflect the presence and intensity of pain7). Non-invasive neuroimaging has been used to demonstrate the effect of analgesic treatment on pain-related brain activation8). Thus, brain activation could be used to uncover LBP mechanisms and suggest treatment strategies5,9).
Chymopapain has been used to treat herniated lumbar intervertebral discs (IVDs). A common adverse effect of chymopapain treatment is LBP10,11). Nonhuman primates are ideal species in which to model clinical LBP because they have IVDs that are anatomically similar to those of humans and undergo biomechanical stress comparable to that of human IVDs. In addition, nonhuman primates have pain processing brain areas similar to those of humans2). The current study developed a behavioral model of chymopapain-induced discogenic LBP in cynomolgus macaques and identified stimulation-evoked brain activation using fMRI.
Materials and Methods
Animals
This study was approved by the institutional review board and followed the relevant guidelines and laws of Japan pertaining to the care and use of laboratory animals. Eight young adult male cynomolgus macaques (weight range: 3.7-4.3 kg; EBS Co., Ltd., Hashimoto, Japan) were used. Animals were individually housed in stainless steel cages in an AAALAC International-accredited primate unit where housing conditions and care were in accordance with the Guide for the Care and Use of Laboratory Animals, Eighth Edition (National Research Council). A 12-hour light-dark cycle was used. Animals were fed a standard diet (Oriental Yeast Co., Ltd., Chiba, Japan) and had free access to water. While individually housed, aural, visual, and olfactory contact with conspecifics was maintained. Manipulanda were provided to each animal. Animals were hand-fed fresh fruits, vegetables, and treats by the animal care and research staff at least once per week. Animals were divided into three groups (control: n = 4; aspiration only: n = 2; chymopapain-treated: n = 2).
Behavioral testing
Pressure thresholds were quantified using a modified pressure algometer (Matsumiya Medical Co., Ltd., Tokyo, Japan). The 9 mm rubber tip of the algometer was covered by a flat plastic circular cap 2.5 cm in diameter. With the macaque standing in a monkey walker, the algometer was placed 2 cm to the right of back midline at the level of L4/L5. The algometer was pushed on the back until the animal responded with contraction of the muscles on the top of the head and around the eyes. Three response pressures (in kg) were measured at 1 min intervals by a blinded investigator, and the mean response pressure was calculated. A cut-off threshold of 3 kg was used.
Intervertebral treatment procedure and postoperative pain time course
Animals were anesthetized with an intramuscular injection of ketamine (10 mg/kg; Daiichi Sankyo Co. Ltd., Tokyo, Japan). Body temperature during the procedure was maintained using a heating pad. Deep anesthesia was ensured when animals did not respond to noxious digit pinch and showed no corneal reflex. Hair on the lower back at the surgical site was shaved and the skin was scrubbed with povidone-iodine. The monkeys were placed in a left semi-lateral decubitus position. Anteroposterior and lateral views of the lumbar spine were obtained using fluoroscopy (SIREMOBIL Compact L, Siemens Healthcare, Berlin, Germany). Unlike humans, there are six lumbar IVDs in cynomolgus macaques. It was safe and easy to perform percutaneous puncture of the IVD between L4/L5 under fluoroscopy without influence of pelvic position. The IVD between L4/L5 was identified and percutaneous puncture of the IVD, with an 18-gauge needle attached to a syringe, was made via an anterolateral retroperitoneal approach directly over the mid-lateral aspect of the disc12). The needle tip was centered within the nucleus pulposus, and the nucleus pulposus was aspirated (n = 2). In macaques treated with chymopapain (n = 2), the nucleus pulposus was aspirated and 0.3 mL chymopapain (1.0 mg/mL in saline; Sigma Chemical Co., St. Louis, MO) was injected. Following recovery from anesthesia, all animals showed normal levels of activity and feeding in their home cage. Response pressure was measured 1, 3, 9, and 14 days after treatment.
Lumbar IVD pathology and visualization of brain activation
T1- and T2-weighted sagittal and axial images of the lumbar vertebrae were obtained using an 3.0T MRI system (Signa HDxt 3.0T MRI system [GE Healthcare, Milwaukee, WI, USA]) (slice thickness, 3 mm; T1-weighted image, TR/TE = 320/15 ms; T2-weighted image, TR/TE =2800/104 ms). The MRI signal intensities, and the IVDs' structure and height, were graded at each time point with the Pfirrmann grading system13). Grading was performed on T2-weighted mid-sagittal IVD images by three orthopedic surgeons who were unaware of the treatment of each vertebra.
Brain activation, without and with low back stimulation, was visualized in four sedated macaques before and 1, 3, 9, and 14 days after treatment using fMRI. Anatomical MRI protocols consisted of a T1-weighted fast spoiled gradient-recalled sequence [repetition time (TR)/echo time (TE), 15.8/7.0 ms; number of averages, 1; flip angle, 12°; field of view, 150 mm × 150 mm; matrix, 256 × 224; slice thickness/interval, 1.0/0.5 mm; number of slices, 168]. Functional scan sequences consisted of field-echo and echo-planar imaging (TR/TE, 3000/35 ms; flip angle, 90°; field of view, 140 mm × 140 mm; matrix, 64 × 64; slice thickness, 2.0 mm; number of slices, 30). The macaques were sedated by continuous intravenous infusion of propofol (0.2 mg/kg/min), which has little analgesic effect14).
To deliver pressure stimuli within the MRI, a disposable, sealed, air-filled 10-mL syringe was applied by hand to the back15). Manual compression on the syringe perpendicularly against an object displaced the plunger, the force of which was indicated on the side of the syringe sheath. In the MRI scanner, macaques were placed in the prone position and pressure was applied to the lower back - the same area that was tested in awake macaques. During one fMRI scan, animals underwent two sets of low back pressure stimulations. One set included ten cycles, with each cycle consisting of 30 s of 1.0 kg followed by 30 s of 0.1 kg. A 30-second interval without stimulation separated each set.
MRI brain data analysis
All MRI analyses were conducted using SPM12 software (Wellcome Trust Centre for Neuroimaging, London, UK). The images were realigned and re-sliced onto mean echo-planar images (EPIs) to correct for head motion. EPIs were co-registered to the corresponding T1-weighted anatomical image and normalized to a macaque brain template16). The resulting images were smoothed with a 4 mm × 4 mm × 4 mm full-width at half-maximum Gaussian kernel. Voxel-wise statistical analysis was based on a general linear model. A fixed-effect model was used for group analysis of data. Contrast (1.0 kg stimulation - 0.1 kg stimulation) was defined to isolate regions responsive to 1.0 kg stimulation-related signals of the whole brain. Brain regions with high signals were selected for analysis. Peak voxels were considered significant at Z score > 1.96 (P < 0.05, uncorrected for multiple comparisons, one-tailed t-test). Brian activation was averaged from two macaques treated with chymopapain and from two macaques with aspiration only of the IVD.
Statistical analysis
Statistical analyses were conducted using SPSS version 23.0 (IBM, Armonk, NY, USA). Continuous variables with normal distributions were expressed as means ± standard deviations (SD) and analyzed using unpaired t-tests. P values < 0.05 were considered statistically significant. While no sample size calculation was used to determine the minimum number of animals needed, the lowest possible number of animals was used to obtain behavioral data. In a previous, unrelated study, a significant decrease in the mean pressure hypersensitivity of the knee joint, following unilateral meniscectomy, was observed between three experimental and three sham-operated animals17).
Results
Following either aspiration or chymopapain treatment, there were no general signs of chronic stress or pain, such as appetite loss or decreased activity. Lumbar MRIs showed slight bleeding in the muscles surrounding the IVD due to the puncture. However, no leakage of chymopapain into the vertebrae or muscles around the disc was observed.
Pressure sensitivity in macaques with chymopapain-induced LBP
Following treatment of the IVD with chymopapain, macaques demonstrated decreased response pressure thresholds (Fig. 1). One day following chymopapain treatment, the response pressure was 0.6 ± 0.05 (mean ± SD) kg, which was significantly less than that of the untreated controls (n = 4; 1.4 ± 0.1 kg; P < 0.01). The response pressure gradually increased over time following chymopapain treatment - 14 days following chymopapain treatment, the response pressure was 1.2 ± 0.04 kg (P < 0.05 vs. untreated controls). By contrast, aspiration of the nucleus pulposus alone did not significantly alter the response to pressure (Fig. 1; P = 0.297 vs. untreated controls).
Figure 1.
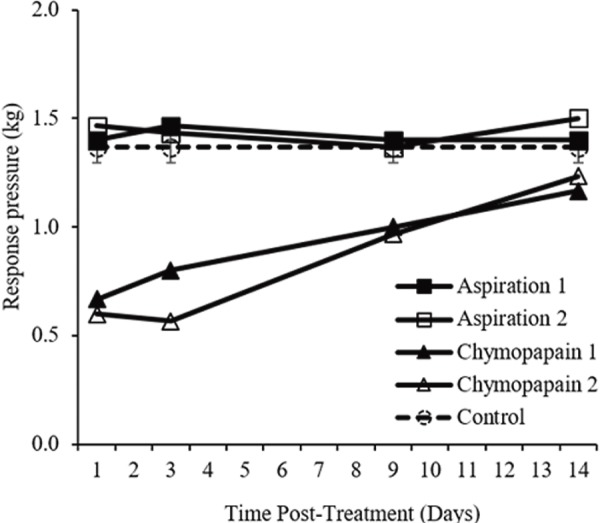
Pressure sensitivity of the lumbar back following chymopapain treatment. Response pressure thresholds (kg) were measured over time after either chymopapain treatment or aspiration of the intervertebral disc. Pressure thresholds of untreated (“control”) macaques are expressed as mean±standard deviation.
IVD pathology following treatment
Treatment with either chymopapain or aspiration alone did not lead to significant changes to the vertebral body; pathology was limited to the IVD. No lumbar disc degeneration was noted in any macaque before treatment (Pfirrmann grade I, Table 1). Beginning one day after nucleus pulposus aspiration, the disc became inhomogeneous, the distinction between the nucleus and annulus was not clear, and the disc height was normal (Pfirrmann grade III; Table 1; Fig. 2). IVD morphology stabilized over time; the Pfirrmann grades 3 to 14 days after aspiration were all III. A similar degradation of the IVD was observed in a macaque treated with chymopapain beginning one day after treatment (Pfirrmann grade III; Table 1; Fig. 3). In the other macaque treated with chymopapain, beginning one day after treatment, the Pfirrmann grade of the IVD was IV and there was a loss of distinction between the nucleus and annulus and a slightly decreased disc height. Disc pathology remained at grade IV for the duration of the observational period.
Table 1.
Change in Lumbar Intervertebral Disc Pathology Over Time According to the Pfirrmann Grading System.
| Case | Before | Day 1 | Day 3 | Day 9 | Day 14 |
|---|---|---|---|---|---|
| Aspiration 1 | I | III | III | III | III |
| Aspiration 2 | I | III | III | III | III |
| Chymopapain 1 | I | IV | IV | IV | IV |
| Chymopapain 2 | I | III | III | III | III |
Figure 2.
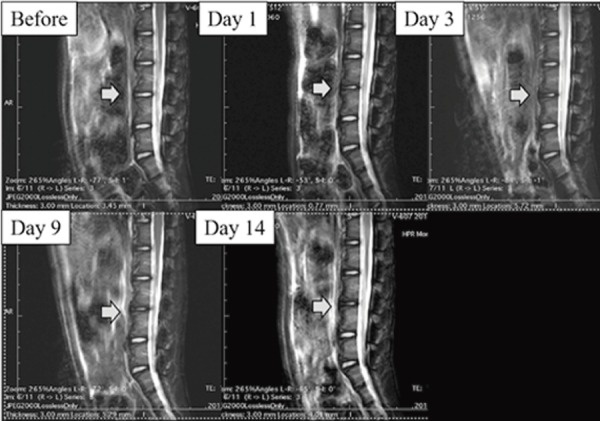
Lumbar magnetic resonance image from a macaque that underwent aspiration only. Beginning one day after aspiration, the distinction between the nucleus and anulus was unclear (Pfirrmann grade III). The disc pathology appeared stable. Gray arrows show the intervertebral discs between L4/L5.
Figure 3.
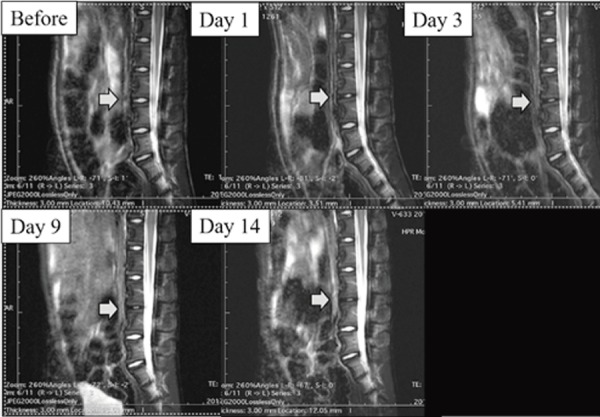
Lumbar magnetic resonance image from a chymopapain-treated macaque. Beginning one day after chymopapain treatment, the distinction between the nucleus and anulus was unclear (Pfirrmann grade III). The disc pathology appeared stable. Gray arrows show the intervertebral discs between L4/L5.
Brain activation
Prior to chymopapain treatment, 1 kg of pressure applied to the back did not evoke significant brain activation in the macaques (Fig. 4A). One (Fig. 4B) and three days after chymopapain treatment, pressure-evoked activation of the secondary somatosensory cortex (SII) and insular cortex (Ins) was observed (Fig. 5; Table 2). Thereafter, no significant pressure-evoked activation of SII or Ins was observed. By contrast, in macaques in which the IVD was aspirated, pressure did not evoke significant brain activation (Fig. 6; Table 3).
Figure 4.
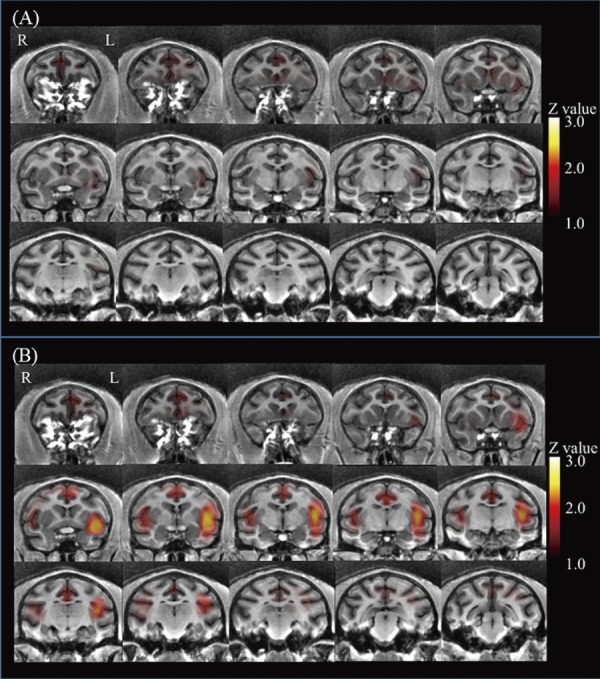
Coronal brain sections arranged from rostral (upper left) to caudal (lower right). Before treatment, there was no significant pressure-induced activation (A). One day after treatment, bilateral pressure-induced activation of the secondary somatosensory cortex and insular cortex was observed (B).
Figure 5.
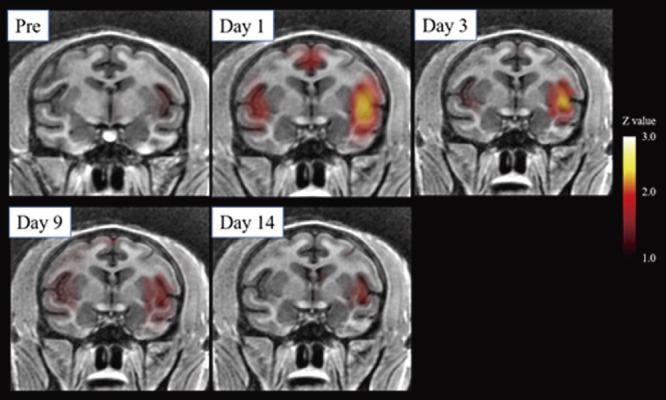
Low back pressure-induced brain activation over time in macaques following chymopapain treatment. Averaged brain images from two macaques treated with chymopapain. Beginning one day after chymopapain treatment, activation of the secondary somatosensory cortex and insular cortex was observed.
Table 2.
Brain Activation during Low Back Pressure Stimulation in Macaques after Chymopapain Treatment Over Time.
| Area | Hemisphere | Z value | Coordinates (mm) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Pretreatment | |||||
| SII and Ins | Right | 0.22 | −16 | 18 | 4 |
| Left | 0.29 | 18 | 16 | 6 | |
| Thalamus | Right | 0.15 | 8 | −2 | 8 |
| Left | 0.17 | −6 | −4 | 8 | |
| Cingulate cortex | 0.24 | 0 | −20 | −10 | |
| Day 1 after chymopapain | |||||
| SII and Ins | Right | 1.98* | −14 | 16 | 4 |
| Left | 2.72* | 16 | 16 | 6 | |
| Thalamus | Right | 0.32 | 4 | −4 | 6 |
| Left | 0.22 | −6 | −2 | 4 | |
| Cingulate cortex | 1.62 | 0 | −22 | −6 | |
| Day 3 after chymopapain | |||||
| SII and Ins | Right | 1.32 | −18 | 16 | 4 |
| Left | 2.41* | 14 | 14 | 4 | |
| Thalamus | Right | 0.31 | 4 | 4 | 6 |
| Left | 0.28 | −6 | 2 | 2 | |
| Cingulate cortex | 0.76 | 2 | −20 | −4 | |
| Day 9 after chymopapain | |||||
| SII and Ins | Right | 1.22 | −14 | 14 | 4 |
| Left | 1.64 | 12 | 10 | 2 | |
| Thalamus | Right | 0.34 | 4 | 2 | 4 |
| Left | 0.36 | −4 | 2 | 2 | |
| Cingulate cortex | 0.43 | 2 | −22 | −6 | |
| Day 14 after chymopapain | |||||
| SII and Ins | Right | 0.78 | −10 | 14 | 4 |
| Left | 1.38 | 14 | 10 | 4 | |
| Thalamus | Right | 0.87 | 6 | 2 | 6 |
| Left | 0.56 | −6 | 4 | 2 | |
| Cingulate cortex | 0.46 | 0 | −24 | −6 | |
1 kg of force was applied to the back of the macaque in a block design during MR imaging.
SII, secondary somatosensory cortex; Ins, insular cortex.
Z values of peak voxels are shown. Stereotaxic coordinates according to Horsley-Clarke’s stereotaxic coordinates. * Peak voxels were considered significant (P <0.05) at Z score >1.96.
Figure 6.
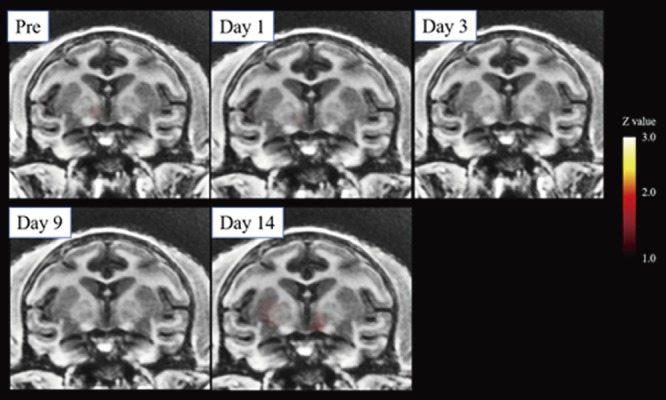
Lack of low back pressure-induced brain activation over time in macaques following aspiration. Averaged brain images over time from two macaques in which the nucleus pulposus was aspirated. There was a lack of significant pressure-evoked brain activation in these macaques.
Table 3.
Brain Activation during Low Back Pressure Stimulation in Macaques before and 1 Day after Aspiration.
| Area | Hemisphere | Z value | Coordinates (mm) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Pretreatment | |||||
| SII and Ins | Right | 0.29 | −18 | 16 | 4 |
| Left | 0.31 | 16 | 16 | 4 | |
| Thalamus | Right | 0.18 | 6 | −2 | 4 |
| Left | 0.22 | −8 | −2 | 6 | |
| Cingulate cortex | 0.30 | 0 | −22 | −4 | |
| Day 1 after aspiration | |||||
| SII and Ins | Right | 0.56 | −16 | 16 | 4 |
| Left | 0.48 | 18 | 14 | 4 | |
| Thalamus | Right | 0.29 | 6 | −2 | 6 |
| Left | 0.22 | −6 | −4 | 8 | |
| Cingulate cortex | 0.28 | 2 | −24 | −4 | |
1 kg of force was applied to the back of the macaque in a block design during MR imaging.
SII, secondary somatosensory cortex; Ins, insular cortex.
Z values of peak voxels are shown. Stereotaxic coordinates according to Horsley-Clarke’s stereotaxic coordinates. Peak voxels were considered significant (P <0.05) at Z score >1.96.
Discussion
The current findings outlined a potential nonhuman primate model of LBP, characterized by low back hypersensitivity to pressure, following IVD treatment with chymopapain. In addition, increased SII and Ins activation during low back stimulation was observed. Interestingly, despite notable and persistent pathology of the IVD, neither low back hypersensitivity nor brain activation was observed beyond three days after treatment. While aspiration of the nucleus pulposus alone led to observable pathology of the IVD comparable to that following chymopapain treatment, there was no change in low back pressure sensitivity or significant brain activation during pressure stimulation. The current findings suggest that chymopapain treatment activates a pain-related mechanism that is not activated by loss of the nucleus pulposus, which further suggests that mechanisms in addition to, or other than, IVD degeneration underlie LBP.
Understanding the mechanism of LBP is hindered in part by a lack of preclinical models that recapitulate clinical LBP2). In general, other species that have been used to model LBP, such as rodents, differ from humans with respect to overall anatomy, movement biomechanics, and molecular and immunologic responses to injury2,18). Thus, the mechanism mediating LBP in rodents may not entirely apply to clinical LBP. More specifically, models of IVD damage in nonhuman primates, a species that is phylogenetically closer to humans than rodents, demonstrated significant IVD pathology but did not measure pain-related behavior2,12,18). Thus, it is unknown whether IVD injury in nonhuman primates leads to LBP.
In order to address this, the current study utilized a pressure algometer to assess pain-related behavior objectively. Quantitative sensory testing (QST) is a clinical method of objectively measuring pain based on a patient's response to graded stimuli19). A key strength of QST is that a particular stimulus may be used to test the functioning of particular aspects of the somatosensory system, thereby potentially suggesting the mechanism. In addition, responsiveness to standardized stimuli could be used to suggest a common mechanism and treatment across patients. Pressure stimulation has been used to assess clinical LPB and demonstrated that the patients had an increased sensitivity to pressure20,21). While other methods of assessing pain-related behavior have been used in preclinical models of LBP, such as spontaneous activity, these tend to be subjective, indirect measures of pain-related behavior22,23). A direct measure of pain was used in the current study, but it is possible that other behavioral indications of acute LBP could be obtained from the macaques.
A further barrier in understanding the mechanism of LBP and the development of effective treatments is elucidating the relationship between pain-related behaviors and central functioning24,25). In vivo neuroimaging has been suggested as an objective method of observing pain by quantification of brain activation with defined stimuli15). Previous fMRI studies involving pressure-induced pain in LBP patients demonstrated activation of pain-related brain nuclei such as the primary somatosensory cortex (SI), SII, posterior cingulate cortex, and Ins4,15). In the current study, activation was observed only in SII and Ins. Some reports have shown that the use of anesthesia could inhibit the detection of pain-related brain activation26,27). Nakamura et al. have reported on the significant differences of brain blood flow in patients with chronic LBP and acute LBP detected by brain SPECT28). The lack of activation of other nuclei in the current study could be due to propofol sedation. The propofol dose used in the current study was high enough to impair movement but not entirely block cortical pain signaling14). Generally, the current macaque model demonstrates activation of brain regions similar to that of patients with LBP. The current findings are the first to demonstrate stimulus-evoked brain activation in a preclinical LBP model. Future studies could be performed on trained awake macaques to uncover possible “resting state” pain that was masked by propofol sedation.
In the current study, chymopapain-induced LBP, whereas aspiration alone did not induce LBP, even though the extent of IVD pathology was similar to that following chymopapain treatment12). A possible explanation for this is that chymopapain induces prolonged inflammation whereas aspiration alone does not2,10,11). It is possible that chymopapain led to an inflammation in tissues outside of the IVD, thereby activating sensory and sympathetic nerves innervating the IVD and vertebrae29). Previous preclinical models of LBP reported a transient yet marked inflammation following injury2,18), which appears in clinical LBP as well30). In the current model, however, no signs of marked inflammation were apparent. Alternatively, it is possible that chymopapain or its breakdown products may have activated peripheral nerves. Further study is needed to identify the mechanism of chymopapain-induced LBP. Understanding why marked IVD pathology alone did not lead to pain in the macaques could assist in understanding a similarly observed clinical finding of a lack of correlation between pathology and pain.
One limitation of the current study was that histopathology was not performed, which may facilitate further understanding of the pain mechanisms in the current model. The distribution of sensory and sympathetic nerves and possible inflammatory mediators in the IVD and vertebral body, which are beyond the detection capability of MRI, could be important in the generation of LBP. Furthermore, a longitudinal study may be needed as peak pain was observed three days after chymopapain treatment but not thereafter, even though marked IVD pathology was observed up to two weeks after treatment. Examination of inflammatory substances during and after peak pain could reveal further clues on the role of the immune system in the maintenance of LBP in the current model and could suggest a mechanism of LBP and potential treatment strategies. The second limitation is usage of only male animal models. Young adult male cynomolgus macaques for assessment of pain were used in the previous study because young adult female cynomolgus macaques might have menstrual pain. The final limitation was that the number of the models was too small. This is a preliminary study for development of LBP animal model, and we hope to conduct future research.
Conflicts of Interest: There are no relevant financial interests outside of the current study. H.T., Y.A., S.O., and T.N. are employees of Hamamatsu Pharma Research, Inc.
Sources of Funding: The current study was supported in part by Hamamatsu University School of Medicine and Hamamatsu Pharma Research, Inc.
Author Contributions: Dr. Matsuyama supervised the study. Dr. Yoshida was responsible for the study's conception and design. Dr. Ushirozako acquired, analyzed, and interpreted data, drafted the article, and approved the final version on behalf of all authors. All authors critically revised the article and reviewed the submitted version.
Acknowledgement
The authors deeply appreciate the expert animal care provided by the Hamamatsu Pharma Research, Inc. Animal Care Group during the course of the current study.
References
- 1.Takahashi N, Kikuchi S, Konno S, et al. Discrepancy between disability and the severity of low back pain: demographic, psychologic, and employment-related factors. Spine (Phila Pa 1976). 2006;31(8):931-9; discussion 40. [DOI] [PubMed] [Google Scholar]
- 2.Ohtori S, Inoue G, Miyagi M, et al. Pathomechanisms of discogenic low back pain in humans and animal models. Spine J. 2015;15(6):1347-55. [DOI] [PubMed] [Google Scholar]
- 3.deCharms RC, Maeda F, Glover GH, et al. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci U S A. 2005;102(51):18626-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50(2):613-23. [DOI] [PubMed] [Google Scholar]
- 5.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konno SI, Sekiguchi M. Association between brain and low back pain. J Orthop Sci. 2018;23(1):3-7. [DOI] [PubMed] [Google Scholar]
- 7.Upadhyay J, Anderson J, Schwarz AJ, et al. Imaging drugs with and without clinical analgesic efficacy. Neuropsychopharmacology. 2011;36(13):2659-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kregel J, Meeus M, Malfliet A, et al. Structural and functional brain abnormalities in chronic low back pain: a systematic review. Semin Arthritis Rheum. 2015;45(2):229-37. [DOI] [PubMed] [Google Scholar]
- 9.Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26(47):12165-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benoist M. [20 years of lumbar chymonucleolysis]. Presse Med. 1996;25(16):743-5. French. [PubMed] [Google Scholar]
- 11.Benoist M, Bonneville JF, Lassale B, et al. A randomized, double-blind study to compare low-dose with standard-dose chymopapain in the treatment of herniated lumbar intervertebral discs. Spine (Phila Pa 1976). 1993;18(1):28-34. [DOI] [PubMed] [Google Scholar]
- 12.Xi Y, Kong J, Liu Y, et al. Minimally invasive induction of an early lumbar disc degeneration model in rhesus monkeys. Spine (Phila Pa 1976). 2013;38(10):E579-86. [DOI] [PubMed] [Google Scholar]
- 13.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26(17):1873-8. [DOI] [PubMed] [Google Scholar]
- 14.Steinbacher DM. Propofol: a sedative-hypnotic anesthetic agent for use in ambulatory procedures. Anesth Prog. 2001;48(2):66-71. [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi Y, Kurata J, Sekiguchi M, et al. Augmented cerebral activation by lumbar mechanical stimulus in chronic low back pain patients: an FMRI study. Spine (Phila Pa 1976). 2009;34(22):2431-6. [DOI] [PubMed] [Google Scholar]
- 16.Black KJ, Koller JM, Snyder AZ, et al. Atlas template images for nonhuman primate neuroimaging: baboon and macaque. Methods Enzymol. 2004;385:91-102. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa S, Awaga Y, Takashima M, et al. Knee osteoarthritis pain following medial meniscectomy in the nonhuman primate. Osteoarthritis Cartilage. 2016;24(7):1190-9. [DOI] [PubMed] [Google Scholar]
- 18.Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17(1):2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Vliet J, Tieleman AA, Verrips A, et al. Qualitative and quantitative aspects of pain in patients with myotonic dystrophy type 2. J Pain. 2018;19(8):920-30. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko H, Zhang S, Sekiguchi M, et al. Dysfunction of nucleus accumbens is associated with psychiatric problems in patients with chronic low back pain: a functional magnetic resonance imaging study. Spine (Phila Pa 1976). 2017;42(11):844-53. [DOI] [PubMed] [Google Scholar]
- 21.Marcuzzi A, Wrigley PJ, Dean CM, et al. From acute to persistent low back pain: a longitudinal investigation of somatosensory changes using quantitative sensory testing-an exploratory study. Pain Rep. 2018;3(2):e641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyagi M, Ishikawa T, Kamoda H, et al. Assessment of pain behavior in a rat model of intervertebral disc injury using the CatWalk gait analysis system. Spine (Phila Pa 1976). 2013;38(17):1459-65. [DOI] [PubMed] [Google Scholar]
- 23.Olmarker K. Puncture of a lumbar intervertebral disc induces changes in spontaneous pain behavior: an experimental study in rats. Spine (Phila Pa 1976). 2008;33(8):850-5. [DOI] [PubMed] [Google Scholar]
- 24.Hama A, Natsume T, Ogawa S, et al. Pain-related behavior and brain activation in a cynomolgus macaque model of postoperative pain. CNS Neurol Disord Drug Targets. 2018;17(5):348-60. [DOI] [PubMed] [Google Scholar]
- 25.Nagasaka K, Yamanaka K, Ogawa S, et al. Brain activity changes in a macaque model of oxaliplatin-induced neuropathic cold hypersensitivity. Sci Rep. 2017;7(1):4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seah S, Asad AB, Baumgartner R, et al. Investigation of cross-species translatability of pharmacological MRI in awake nonhuman primate - a buprenorphine challenge study. PLoS One. 2014;9(10):e110432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lahti KM, Ferris CF, Li F, et al. Comparison of evoked cortical activity in conscious and propofol-anesthetized rats using functional MRI. Magn Reson Med. 1999;41(2):412-6. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura Y, Nojiri K, Yoshihara H, et al. Significant differences of brain blood flow in patients with chronic low back pain and acute low back pain detected by brain SPECT. J Orthop Sci. 2014;19(3):384-9. [DOI] [PubMed] [Google Scholar]
- 29.Suseki K, Takahashi Y, Takahashi K, et al. Sensory nerve fibres from lumbar intervertebral discs pass through rami communicantes. A possible pathway for discogenic low back pain. J Bone Joint Surg Br. 1998;80(4):737-42. [DOI] [PubMed] [Google Scholar]
- 30.Burke JG, Watson RW, McCormack D, et al. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84(2):196-201. [DOI] [PubMed] [Google Scholar]


