Abstract
In this study, the molecular mechanism for inhibitory effect of Catalpol coordinated with Budesonide (BUD) on allergic airway inflammation was investigated. A total of 30 adult SD rats were randomly divided into five groups, namely the positive control group, the model group, the Catalpol group, the BUD group, and the Catalpol+BUD group with 6 rats in each group, respectively. The pathologic changes of lung tissue were observed by HE stain method. The lung function of rats, the cell count, and the cytokine concentrations in bronchoalveolar lavage fluid (BALF) were detected. The levels of cytokines [interleukin-4 (IL-4), interleukin-5 (IL-5), and interferon gamma (IFN-γ)] in BALF were measured using enzyme-linked immunosorbent assay method. The expressions of Interleukin-13 (IL-13) and Eotaxin in lung tissue were measured by RT-PCR method. The total number of cells in the BALF of the group treated with Catalpol and BUD was significantly lower than the model group. The cytokines IL-5 and IL-4 exhibited a similar tendency: the concentrations of IL-4 and IL-5 for the Catalpol group were dramatically decreased compared with the model group. However, the IFN-γ concentration for the Catalpol and BUD groups were higher than the model group. After treatment with Catalpol+BUD, the eosinophils and neutrophils of the rats were further reduced, asthma-associated inflammation was obviously inhibited, IL-4 level was further decreased and IFN-γ level was further increased comparing the Catalpol group and the BUD group. Moreover, IL-13 expression was positively correlated with Eotaxin expression. The results indicated that Catapol could inhibit the expression of IL-13 and Eotaxin in the lung of asthmatic rats, which also exhibited a synergistically inhibitory effect with BUD on airway inflammation. It is suggested that Catalpol+BUD might be an effective and potential treatment for the clinical therapy of asthma.
Keywords: Catalpol, budesonide, asthma, IL-13, cytokines
Introduction
Bronchial asthma, in short asthma, is a chronic inflammation of bronchi. Disrupted homeostasis of various immune cells, such as helper T cells, eosinophils, mast cells and neutrophils have been commonly observed in asthma [1-4]. The primary changes in pathology result from inflammatory reactions of bronchial mucous membrane, hyperkinesia of bronchial smooth muscle and a reversible block of bronchi resulting from secretion [5-9]. During asthma exacerbation, IgE produced by immunocyte has been considered as the most important factor of the disease when the immune system was activated by allergen [10,11]. Inflammatory reactions of respiratory tract are the fundamentals of hypersensitivity. Many inflammatory cells such as neutrophil macrophage, eosinophil, T cell and B cell, inflammatory medium and cytokine participate in the inflammatory reactions [12-15]. Therefore, the methods of treatment of asthma should include the control of airway inflammation, the elimination of airway edema, the reduction of mucus secretion, and the improvement of the immune function of the body.
Catalpol (C15H22O10) belongs to one of 70 monomeric compounds that have been separated from Radix Rehmanniae Preparata (Shudihuang) - a common traditional Chinese herbal medicine for nourishing the kidney according to the traditional Chinese medicine theory [16-20]. In previous studies [21,22], we have demonstrated that the use of large doses of Catalpol injection can significantly mitigate the symptoms of asthma in an OVA-induced rat model, Catalpol is able to inhibit eosinophil infiltration in the asthmatic rats, which may be considered as the anti-asthmatic effect of Catalpol. Furthermore, we showed that after Catalpol treatment, the level of IFN-γ the in the serum of rats significantly increased, but the level of IL-4 decreased, suggesting that Catalpol improved the balance of the ratio IL-4/IFN-γ in asthma. These results and findings indicate that Catalpol may become a promising drug for the treatment of asthma. However, there are little to no studies on the mechanism of synergistic effect of Catapol and other methods in the treatment of asthma.
Budesonide (BUD) is a glucocorticoid with high local anti-inflammatory effect [23-32]. BUD can enhance the stability of endothelial cells, smooth muscle cells and lysosome membranes, inhibit immune response and reduce antibody synthesis, thus reducing the release and activity of allergic active mediators such as histamine. BUD can also alleviate the enzymatic process stimulated by antigen-antibody binding, and inhibit the synthesis of bronchial contractile substances to release and alleviate contractile response of smooth muscle [33-35]. BUD has been clinically used in patients with glucocorticoid-dependent or non-dependent bronchial asthma and chronic asthmatic bronchitis [36-40]. The chemical structures of Catalpol and BUD are shown in Figure 1.
Figure 1.
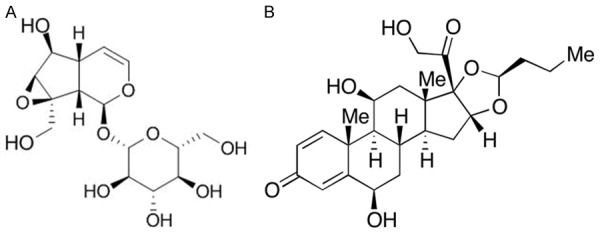
Chemical structures of (A) Catalpol and (B) BUD.
In this study, an asthmatic model was established using SD rats, a coordinated effect of Catalpol and BUD on asthmatic rats was investigated by cell classification and counting in bronchoalveolar lavage fluid (BALF) and serum ovalbumin (OVA). Interleukin 13 (IL-13) and Eotaxin are important factors in the pathogenesis of asthma. The expression of IL-13 and Eotaxin in the lungs of asthmatic rats and the changes of eosinophils in BALF were observed. The molecular mechanism of Catalpol combined with BUD in inhibiting airway allergic inflammation in bronchial asthma was probed and discussed. The present study demonstrates that the therapeutic effects of Catalpol on asthma can be significantly enhanced by coordination with BUD in a rat model of OVA-induced asthma.
Materials and methods
Experimental animals
Thirty SPF grade male SD rats (age: 5-6 weeks, weight: 170-200 g) were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). The rats were divided into five groups-the positive control group, the model group, the Catalpol group, the BUD group, and the Catalpol+BUD group with 6 rats in each group. Temperature and humidity were 25 ± 1°C and 55%-60%, respectively. Illumination time and dark time were both 12 h. There were no restrictions on drinking water and feeding. The care and treatment of the animals were approved by the Animal Care and Use Committees of Heilongjiang University of Chinese Medicine.
Chemicals and reagents
OVA was purchased from Sigma-Aldrich (St. Louis, MO, USA), Catalpol injectable powder was purchased from Melone Pharmaceutical Co., Ltd (Dalian, China), BUD was manufactured by CR double-crane Pharmaceutical Co Ltd (Beijing, China), aluminum hydroxide (99.9%) was purchased from Sinopharm (Shanghai, China), and the corresponding enzyme-linked immunosorbent assay (ELISA) kits were purchased from USCN and Boster (Wuhan, China). All other chemicals or reagents were purchased from Sinopharm (Beijing, China) without further purification.
Establishment of asthma model
The asthma model was established by sensitization and challenge with OVA. Briefly, the rats were sensitized with 1% OVA (10 mg OVA and 200 mg aluminum hydroxide) in 1 mL of normal saline via intraperitoneal injection on Day 1 and Day 8. From Day 15 to Day 28, the rats were challenged with 1% OVA for 30 min every day using an ultrasonic nebulizer. The rats in the Control group received an equal amount of normal saline. From Day 15 to Day 28, the rats in the Catalpol group and the BUD group daily received a dose (10 mg/kg in normal saline) of Catalpol and a dose (1 mg/kg) of BUD, respectively, through intraperitoneal injection 0.5 h prior to each OVA challenge, and the rats in the Catalpol+BUD group received a joint treatment combining 10 mg/kg Catalpol and 1 mg/kg BUD via intraperitoneal injection 0.5 h prior to each OVA challenge. The rats in the control group and experimental groups received equal amounts of normal saline. On Day 29, the peripheral blood and BALF in each group were harvested, and the lung tissues were also collected.
Cell count and classification of BALF
All the rats were executed after last OVA challenge. Normal saline was injected into the right ventricle to completely remove the residual blood in the lung. The lung was taken from the chest cavity after washing. A tube was inserted into the syringe to inject 1 mL of PBS solution for bronchoalveolar lavage three times. BALF was obtained, and the total number of cells was counted with 10 µL BALF. BALF was centrifuged for 10 minutes at 1500 rpm. The supernatant was extracted for cytokines detection, and the cells were classified and counted after precipitation and suspension to determine the percentages of neutrophils, eosinophils, lymphocytes, and macrophages.
HE staining
The right lung tissues were placed and fixed in 4% polyformaldehyde followed by dehydration using gradient ethanol. The tissues were embedded, fixed, sectioned then stained using HE method.
Determination of IgE and cytokines
The level of IgE in the BALF was determined using an IgE ELISA kit. The levels of IL-4, IL-5, and IFN-γ in the BALF were determined using the ELISA kits according to the manufacturer’s instructions. In the measurement, the optical density (OD) values were recorded using a Biorad450 enzyme standard instrument at the wavelength of 450 nm. OD values more than twice of the negative control were defined as positive.
mRNA determination of lung tissue IL-13
The content of lung tissue was determined by reverse transcription-polymerase chain reaction (RT-PCR). The central tissue of left lung (100 mg) was taken, and the total RNA was extracted by adding Trizol. The purity and content of RNA were determined by UV spectrophotometer. RNA (5 μg) was used to synthesize cDNA under the presence of oligo-dT and M-Mulv. After amplification of 35 cycles according to their respective reaction conditions, PCR products were electrophoretic on 1% agarose gel, and β-actin was used as positive control. Imaging and strip density scanning were conducted by gel imaging analysis system and semi-quantitative analysis was performed with integral optical density ratio IODIL-13/IODβ-actin.
Statistical methods
The experimental data were analyzed by SPSS17.0 software. Single-factor Analysis of Variance (ANOVA) was used to compare the difference between the groups. The least significant difference method was used for the comparison of two sets of data with the homogeneity of variance. Dunnett’s t-test was used for heterogeneity of variance. Spearman’s rank correlation analysis was used for correlation analysis. P < 0.05 indicated that there were no significant differences between the data. The results were expressed by x ± s.
Results
Determination of IgE in BALF
The level of IgE in BALF was determined by the ELISA method. As shown in Figure 2, the level of IgE in BALF was increased in the model group, and it was significantly higher than that in the control group with P < 0.01. After Catalpol or BUD single treatment, the IgE level was dramatically decreased, and it was significantly lower than that in the model group with P < 0.01. It should be noted that after the combined treatment using Catalpol+BUD, the IgE level was further decreased, and it was significantly lower than that in the Catalpol or BUD group with P < 0.01.
Figure 2.
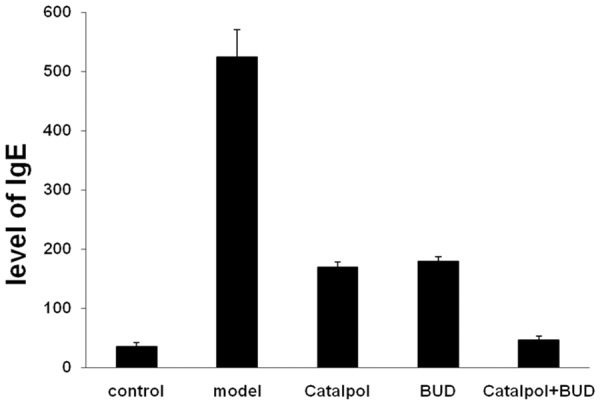
The level of IgE in BALF from each group.
Cell counts in BALF
After the sensitization and OVA-challenge, Figure 3 shows that the total number of cells in the model group was significantly increased comparing the control group with P < 0.01. Figure 3 also indicates that after the Catalpol or BUD treatment, the total number of cells was decreased comparing the model group with P < 0.01. In addition, after the combined treatment using Catalpol+BUD, the total number of cells was further decreased, and it was significantly lower than that in the Catalpol or BUD group with P < 0.01. Figure 4 shows that the percentages of eosinophils and neutrophils in the model group were significantly increased comparing the control group with P < 0.01. Figure 4 also indicates that after the Catalpol or BUD treatment, the percentages of eosinophils and neutrophils were decreased comparing the model group with P < 0.01. In addition, after the combined treatment using Catalpol+BUD, the percentages of eosinophils and neutrophils were further decreased, and it was significantly lower than that in the Catalpol or BUD group with P < 0.01.
Figure 3.
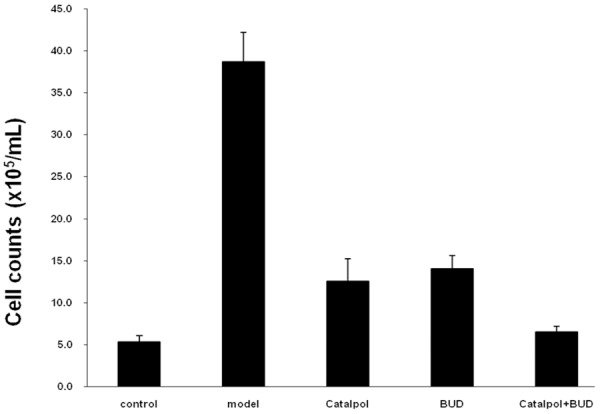
Total number of cells in BALF from each group.
Figure 4.
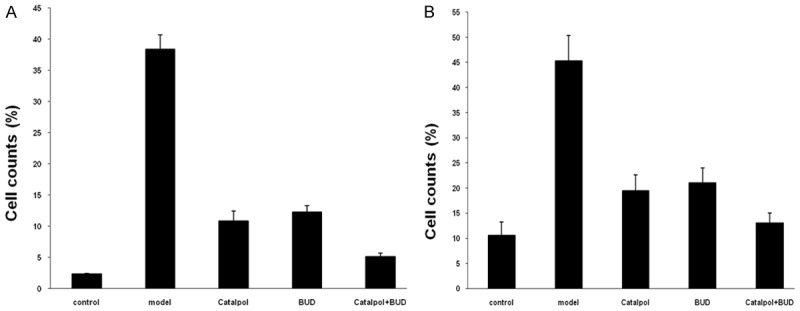
Percentages of (A) eosinophils and (B) neutrophils in BALF from each group.
Concentration of cytokines
The concentrations of cytokine in the BALF from each group are shown in Figure 5. The cytokines IL-4 and IL-5 in the model group were significantly increased comparing the control group with P < 0.05, and the cytokines IL-4 and IL-5 in the Catalpol or BUD group were significantly decreased comparing the model group with P < 0.05. Furthermore, after the combined treatment using Catalpol+BUD, the cytokines IL-4 and IL-5 were further decreased, and it was significantly lower than that in the Catalpol or BUD group with P < 0.01. However, the IFN-γ in the model group was decreased comparing the control group with P < 0.05, IFN-γ in the Catalpol or BUD group was increased comparing the model group with P < 0.05, IFN-γ in the catalpol+BUD group was further increased comparing the Catalpol or BUD group with P < 0.05.
Figure 5.
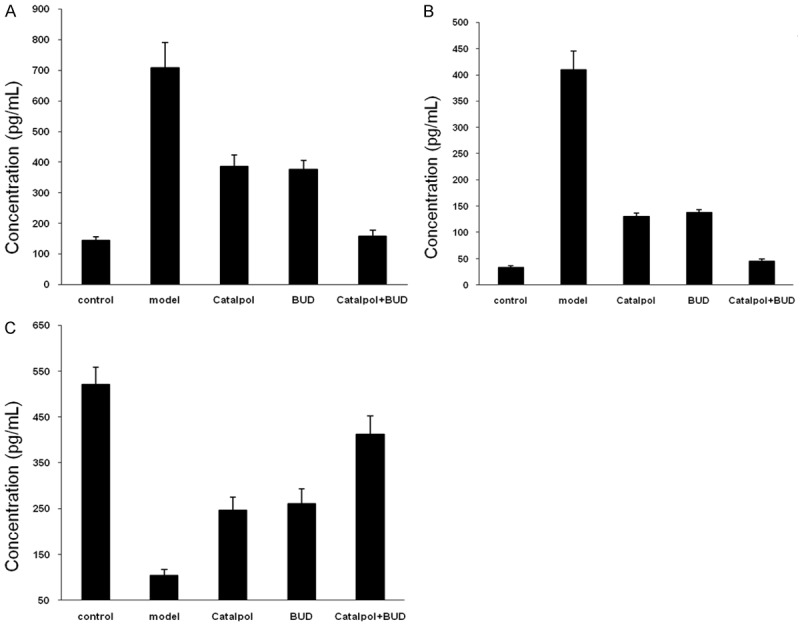
Cytokine concentrations-(A) IL-5, (B) IL-4 and (C) IFN-γ in BALF from each group.
Pathological changes of lung tissue
The inflammatory cells in the lung tissue of rats were observed with light microscopy with H&E staining, as shown in Figure 6. There were no inflammatory changes in the lung tissue in the control group and significant inflammation changes in the model group. Moderate to mild inflammation changes in the Catalpol or BUD group and mild inflammation changes in the Catalpol+BUD group were observed, which were basically close to the normal group, indicating efficient treatment using the selected drugs.
Figure 6.
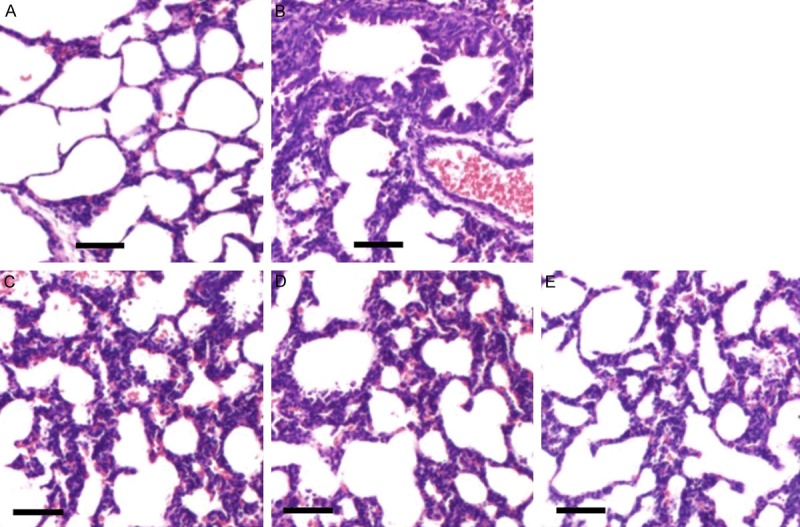
Observation of pathological changes in the lung tissues of rats under the light microscope with H&E staining. (A-E) show the pathological changes of rats in the control group, the model group, the Catalpol group, the BUD group, and the Catalpol+BUD group, respectively (scale bar 100 μm).
Expression of IL-13 mRNA
Figure 7 shows the expression of IL-13 mRNA obtained by RT-PCR method. The expression in the model group was significantly enhanced comparing the control group with P < 0.01. After treatment by Catalpol or BUD, the expression was significantly reduced compared with the model group with P < 0.05, The expression in the Catapol+BUD group was significantly reduced comparing the Catalpol group (P < 0.05) or BUD group (P < 0.01).
Figure 7.
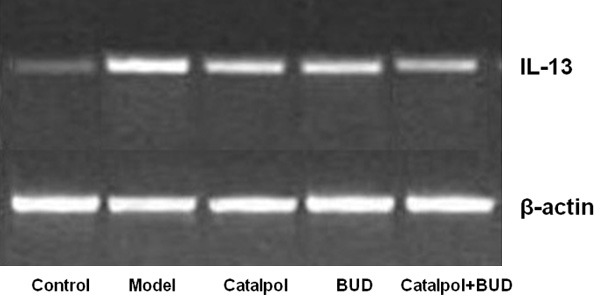
The protein level of IL-13 for each group was detected by PCR method with β-actin as the internal reference.
Discussion
Bronchial asthma is a chronic airway inflammatory disease caused by a variety of cells, cytokines and inflammatory mediators, which is characterized by eosinophil infiltration and airway hyperresponsiveness (AHR) and reversible airway obstruction [1-3]. Cytokines and chemokines play an important role during the formation of asthma inflammation [4-6]. EOS infiltration is the main feature of airway pathology. EOS interaction in T lymphocyte is the central link in the pathogenesis of asthma. The main target cells are Th2 (CD4+) cells to induce EOS inflammation [1-6]. IL-13 is the most important Th2 cytokine that has been investigated in the past five years [7-9]. It has been considered as the most directly related Th2 factor in the pathogenesis of asthma. IL-13 acts as a central function in the pathogenesis of allergic asthma [10-13] and regulates eosinophilic inflammation, mucus secretion and airway hyperresponsiveness. Eotaxin has specific chemotaxis to EOS, such chemotaxis is stronger than other chemotaxis factors [14,15]. Eotaxin is an important pathogenic factor in patients with bronchial asthma [16]. It tends to recruit EOS and other inflammatory cells in bronchial and lung tissues. The expression level of Eotaxin in serum is closely related to the clinical severity of asthma. It has been reported that the expression level of Eotaxin in sputum of asthmatic patients was related to the acute attack of asthma [17-19]. IL-13 and Eotaxin interact with each other in peripheral blood and tissues to regulate the recruitment of EOS and make great contribution to the pathogenesis of asthma [23,24]. IL-13 is a strong inducer of Eotaxin, which can up-regulate the expression of Eotaxin mRNA and protein. IL-13 also stimulates Eotaxin release through mitogen-activated protein and signal transduction activator-6 (STAT6) signal transduction pathway in airway smooth muscle [25,26]. Blocking IL-13 or Eotaxin pathway can be used to reduce the number of EOS and this method exhibits therapeutic effect on bronchial asthma.
Although glucocorticoid such as BUD has been considered as the most effective drug in the treatment of asthma, the current studies showed that BUD was able to down-regulate the expression of IL-13 and Eotaxin, which has significant effects on reducing AHR and inhibiting airway allergy. However, the toxicity and side effects of large doses of hormones were too significant to adopt a long-term treatment. Therefore, an integration of traditional Chinese medicine and western medicine could improve the curative effect by combining their advantages to promote the total therapeutic effects.
Conclusions
In this study, the asthmatic rat model was established, and the total number of white blood cells and EOS in the Catalpol group were significantly reduced comparing the model group after intervention with Catalpol injection and BUD injection. The total number of leukocytes and EOS in BALF of the Catalpol+BUD group were significantly lower than the Catalpol group and the BUD group. These results suggest that Catalpol injection can inhibit airway inflammation in asthmatic rats and enhance the anti-inflammatory effect of BUD. The expression of IL-13 and Eotaxin in lung tissue of Catalpol group was significantly lower than that of model group. The expression of IL-13 and Eotaxin in lung tissue of the Catalpol+BUD group was significantly lower than that of Catalpol group and BUD group, and the expression of IL-13 and Eotaxin was positively correlated. Therefore, it can be speculated that Catalpol injection can combine with BUD to result in a synergistic effet, which down-regulate the expression of IL-13 and Eotaxin in the lung tissue of asthmatic rats and further inhibit the airway inflammation of asthma.
Disclosure of conflict of interest
None.
References
- 1.Braman SS. The global burden of asthma. Chest. 2006;130:4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 2.Asher I, Pearce N. Global burden of asthma among children. Int J Tuberc Lung Dis. 2014;18:1269–1278. doi: 10.5588/ijtld.14.0170. [DOI] [PubMed] [Google Scholar]
- 3.Noble PB, Ansell TK, James AL, McFawn PK, Mitchell HW. Airway smooth muscle dynamics and hyperresponsiveness: in and outside the clinic. J Allergy (Cairo) 2012;2012:157047. doi: 10.1155/2012/157047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai D, Brightling C. Cytokine and anti-cytokine therapy in asthma: ready for the clinic? Clin Exp Immunol. 2009;158:10–19. doi: 10.1111/j.1365-2249.2009.03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wardlaw AJ, Brightling C, Green R, Woltmann G, Pavord I. Eosinophils in asthma and other allergic disease. Br Med Bull. 2000;56:985–1003. doi: 10.1258/0007142001903490. [DOI] [PubMed] [Google Scholar]
- 6.Wardlaw AJ, Brightling CE, Green R, Woltmann G, Bradding P, Pavord ID. New insights into the relationship between airway inflammation and asthma. Clin Sci (London) 2012;103:201–211. doi: 10.1042/cs1030201. [DOI] [PubMed] [Google Scholar]
- 7.Brightling CE, Bradding P, Pavord ID, Wardlaw AJ. New insights into the role of the mast cell in asthma. Clin Exp Allergy. 2003;33:550–556. doi: 10.1046/j.1365-2222.2003.01636.x. [DOI] [PubMed] [Google Scholar]
- 8.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 9.Busse WW, Sedgwick JB. Eosinophils in asthma. Ann Allergy. 1992;68:286–290. [PubMed] [Google Scholar]
- 10.Venarske D, deShazo RD. Molecular mechanisms of allergic disease. South Med J. 2003;96:1049–1054. doi: 10.1097/01.SMJ.0000097887.04639.39. [DOI] [PubMed] [Google Scholar]
- 11.Zhang RX, Li MX, Jia ZP. Rehmannia glutinosa: review of botany, chemistry and pharmacology. J Ethnopharmacol. 2008;117:199–214. doi: 10.1016/j.jep.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Xu Z, Jiang Z, Sun L, Ji J, Miao J, Zhang X, Huang S, Wang T, Zhang L. Hypoglycemic effect of catalpol on high-fat diet/streptozotocin-induced diabetic mice by increasing skeletal muscle mitochondrial biogenesis. Acta Biochim Biophys Sin (Shanghai) 2014;46:738–748. doi: 10.1093/abbs/gmu065. [DOI] [PubMed] [Google Scholar]
- 13.Tian YY, An LJ, Jiang L, Duan YL, Chen J, Jiang B. Catalpol protects dopaminergic neurons from LPS-induced neurotoxicity in mesencephalic neuron-glia cultures. Life Sci. 2006;80:193–199. doi: 10.1016/j.lfs.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Jiang B, Du J, Liu JH, Bao YM, An LJ. Catalpol attenuates the neurotoxicity induced by beta-amyloid (1-42) in cortical neuron-glia cultures. Brain Res. 2008;1188:139–147. doi: 10.1016/j.brainres.2007.07.105. [DOI] [PubMed] [Google Scholar]
- 15.Jin D, Cao M, Mu X, Yang G, Xue W, Huang Y, Chen H. Catalpol inhibited the proliferation of T24 human bladder cancer cells by inducing apoptosis through the blockade of Akt-mediated anti-apoptotic signaling. Cell Biochem Biophys. 2015;71:1349–1356. doi: 10.1007/s12013-014-0355-0. [DOI] [PubMed] [Google Scholar]
- 16.Gao N, Tian JX, Shang YH, Zhao DY, Wu T. Catalpol suppresses proliferation and facilitates apoptosis of OVCAR-3 ovarian cancer cells through upregulating microRNA-200 and downregulating MMP-2 expression. Int J Mol Sci. 2014;15:19394–19405. doi: 10.3390/ijms151119394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bi J, Jiang B, Zorn A, Zhao RG, Liu P, An LJ. Catalpol inhibits LPS plus IFN-γ-induced inflammatory response in astrocytes primary cultures. Toxicol In Vitro. 2013;27:543–550. doi: 10.1016/j.tiv.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J, Xu G, Ma S, Li F, Yuan M, Xu H, Huang K. Catalpol ameliorates high-fat diet-induced insulin resistance and adipose tissue inflammation by suppressing the JNK and NF-kappaB pathways. Biochem Biophys Res Commun. 2015;467:853–858. doi: 10.1016/j.bbrc.2015.10.054. [DOI] [PubMed] [Google Scholar]
- 19.Choi HJ, Jang HJ, Chung TW, Jeong SI, Cha J, Choi JY, Han CW, Jang YS, Joo M, Jeong HS, Ha KT. Catalpol suppresses advanced glycation end-products-induced inflammatory responses through inhibition of reactive oxygen species in human monocytic THP-1 cells. Fitoterapia. 2013;86:19–28. doi: 10.1016/j.fitote.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Park KS. Catalpol reduces the production of inflammatory mediators via PPARgamma activation in human intestinal Caco-2 cells. J Nat Med. 2016;70:620–626. doi: 10.1007/s11418-016-0988-y. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Zhang Y, Xu M, Luan J, Piao S, Chi S, Wang H. Catalpol alleviates ovalbumin-induced asthma in mice: reduced eosinophil infiltration in the lung. Int Immunopharmacol. 2017;43:140–146. doi: 10.1016/j.intimp.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Wang H, Yang X. Effects of catalpol on bronchial asthma and its relationship with cytokines. J Cell Biochem. 2019;120:8992–8998. doi: 10.1002/jcb.28170. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Jin C, Li Y, Guan S, Han F, Zhang S. Catalpol improves cholinergic function and reduces inflammatory cytokines in the senescent mice induced by D-galactose. Food Chem Toxicol. 2013;58:50–55. doi: 10.1016/j.fct.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Chen X, Wang H, Yan Q. Catalpol protects mice against renal ischemia/reperfusion injury via suppressing PI3K/Akt-eNOS signaling and inflammation. Int J Clin Exp Med. 2015;8:2038–2044. [PMC free article] [PubMed] [Google Scholar]
- 25.Wei Y, Luo QL, Sun J, Chen MX, Liu F, Dong JC. Bu-Shen-Yi-Qi formulae suppress chronic airway inflammation and regulate Th17/Treg imbalance in the murine ovalbumin asthma model. J Ethnopharmacol. 2015;164:368–377. doi: 10.1016/j.jep.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Zhang XL, Jiang B, Li ZB, Hao S, An LJ. Catalpol ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by D-galactose. Pharmacol Biochem Behav. 2007;88:64–72. doi: 10.1016/j.pbb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Bi J, Jiang B, Liu JH, Lei C, Zhang XL, An LJ. Protective effects of catalpol against H2O2-induced oxidative stress in astrocytes primary cultures. Neurosci Lett. 2008;442:224–227. doi: 10.1016/j.neulet.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Bao Y, Jiang B, Wang Z, Liu Y, Zhang C, An L. Catalpol protects primary cultured astrocytes from in vitro ischemia-induced damage. Int J Dev Neurosci. 2008;26:309–317. doi: 10.1016/j.ijdevneu.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Fu K, Piao T, Wang M, Zhang J, Jiang J, Wang X, Liu H. Protective effect of catalpol on lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol. 2014;23:400–406. doi: 10.1016/j.intimp.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Durrant DM, Metzger DW. Emerging roles of T helper subsets in the pathogenesis of asthma. Immunol Invest. 2010;39:526–549. doi: 10.3109/08820131003615498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray A, Cohn L. Th2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. J Clin Invest. 1999;104:985–993. doi: 10.1172/JCI8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnes PJ. Th2 cytokines and asthma: an introduction. Respir Res. 2001;2:64–65. doi: 10.1186/rr39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi H, Qin S, Huang G, Chen Y, Xiao C, Xu H, Liang G, Xie Z, Qin X, Wu J, Li G, Zhang C. Infiltration of eosinophils into the asthmatic airways caused by interleukin 5. Am J Respir Cell Mol Biol. 1997;16:220–224. doi: 10.1165/ajrcmb.16.3.9070605. [DOI] [PubMed] [Google Scholar]
- 34.Delphin S, Stavnezer J. Regulation of antibody class switching to IgE: characterization of an IL-4-responsive region in the immunoglobulin heavy-chain germline epsilon promoter. Ann N Y Acad Sci. 1995;764:123–135. doi: 10.1111/j.1749-6632.1995.tb55815.x. [DOI] [PubMed] [Google Scholar]
- 35.Garcia G, Taille C, Laveneziana P, Bourdin A, Chanez P, Humbert M. Antiinterleukin-5 therapy in severe asthma. Eur Respir Rev. 2013;22:251–257. doi: 10.1183/09059180.00004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wegmann M. Targeting eosinophil biology in asthma therapy. Am J Respir Cell Mol Biol. 2011;45:667–674. doi: 10.1165/rcmb.2011-0013TR. [DOI] [PubMed] [Google Scholar]
- 37.Huang WC, Liou CJ. Dietary acacetin reduces airway hyperresponsiveness and eosinophil infiltration by modulating eotaxin-1 and th2 cytokines in a mouse model of asthma. Evid Based Complement Alternat Med. 2012;2012:910520. doi: 10.1155/2012/910520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ok IS, Kim SH, Kim BK, Lee JC, Lee YC. Pinellia ternata, Citrus reticulata, and their combinational prescription inhibit eosinophil infiltration and airway hyperresponsiveness by suppressing CCR3+ and Th2 cytokines production in the ovalbumin-induced asthma model. Mediators Inflamm. 2009;2009:413270. doi: 10.1155/2009/413270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ying S, Robinson DS, Meng Q, Rottman J, Kennedy R, Ringler DJ, Mackay CR, Daugherty BL, Springer MS, Durham SR, Williams TJ, Kay AB. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol. 1997;27:3507–3516. doi: 10.1002/eji.1830271252. [DOI] [PubMed] [Google Scholar]
- 40.Ying S, Meng Q, Zeibecoglou K, Robinson DS, Macfarlane A, Humbert M, Kay AB. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (intrinsic) asthmatics. J Immunol. 1999;163:6321–6329. [PubMed] [Google Scholar]


