Figure 1.
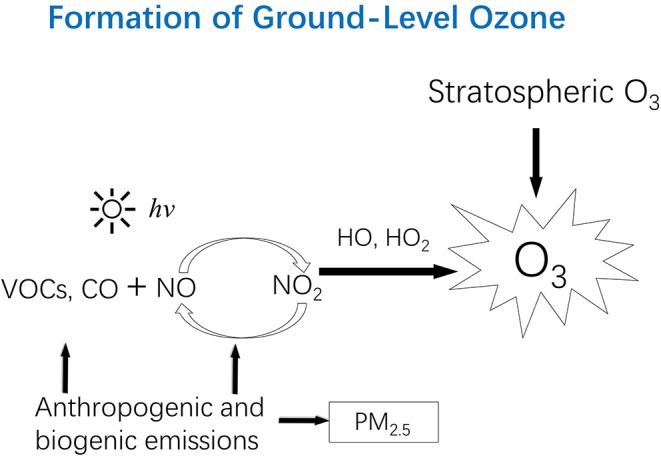
Ozone in the stratosphere can move downward to the troposphere, contributing to the “background” level of ground-level ozone. However, high levels of ozone in the troposphere are due to photochemical reactions involving volatile organic compounds (VOCs) and oxides of nitrogen (NOx: NO, and NO2). Anthropogenic emissions (e.g., fossil fuel combustion) are responsible for NOx and mainly responsible for VOCs and CO. Trees also emit certain VOCs (e.g., isoprene). PM2.5 from primary emission sources can react with (consume) free radicals (e.g., HO2) responsible for ozone formation, which partly explains the observations in certain areas where ozone level increased while PM2.5 level decreased. hv, photon; VOCs, volatile organic compounds; CO, carbon monoxide; NO, nitric oxide; NO2, nitrogen dioxide; NOx, NO and NO2; HO, the hydroxyl radical; HO2, The hydroperoxy radical; PM2.5, Particulate matter with a diameter of 2.5 μm or less.
