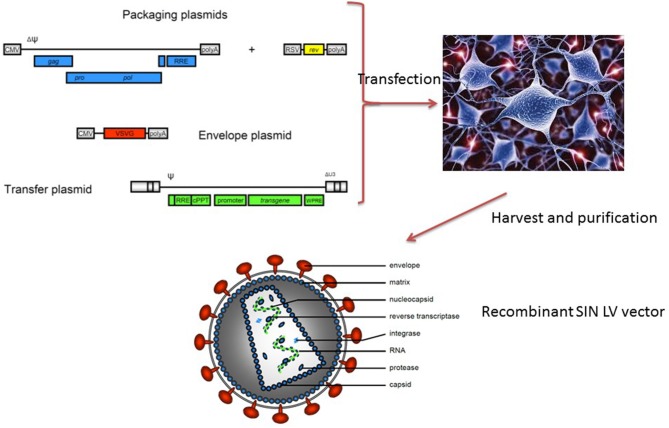
Figure 1.
Current methods for generating lentiviral vectors. Four plasmids (a transfer vector containing the therapeutic gene and viral long terminal repeats, a REV-containing plasmid, a GAG-POL encoding plasmid, and an envelope-encoding plasmid, most often VSV-G) into a packaging line that subsequently secretes replication-deficient lentiviral particles. The latter are purified and then tested in several efficacy and safety assays before clinical use.