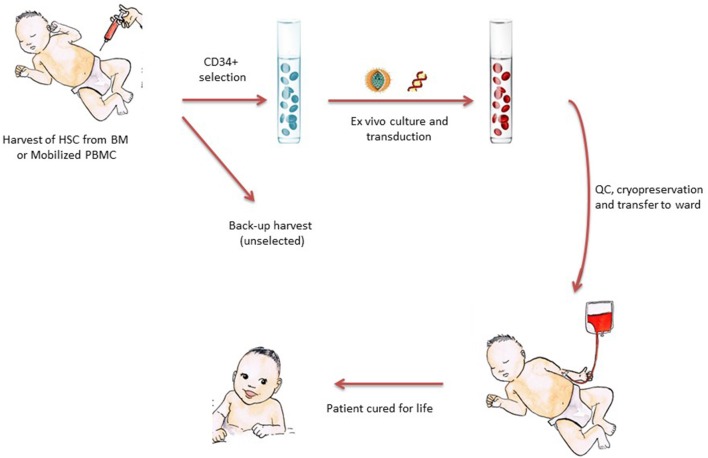
Figure 2.
The principle of stem-cell-based gene therapy for pediatric diseases. CD34+ cells enriched for HSCs are harvested from the patients—either from bone marrow or (increasingly) from mobilized peripheral blood. The CD34+ cells are cultured in GMP laboratories with cytokines and viral vectors, harvested, and then subjected to a number of quality control steps prior to reinfusion into the patient. The cell product is often cryopreserved to allow time for quality control tests and the shipment of cells to clinical transplantation centers far from the production site. After reinfusion, HSCs find their niches, differentiate into mature blood cells, and thereby restore the clinical defect.