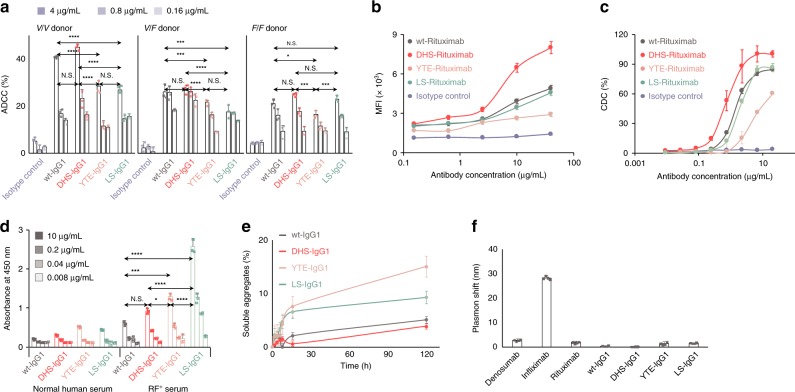
Fig. 3.
Features of DHS Fc variants relevant to therapeutic antibody development. a ADCC assay of SK-BR-3 with PBMCs from FcγRIIIa V/V, V/F, or F/F donors. b C1q deposition on CD20+ Raji cells revealed by flow cytometry. c CDC assay of Rituximab-Fc variants with Raji cells as a function of antibody concentration. d Binding to rheumatoid factor (RF) measured by ELISA. P values by two-way ANOVA test, NS P ≥ 0.05, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001. e Extent of antibody aggregation following thermal stress (50 °C incubation) of Trastuzumab variants, measured by size exclusion chromatography (SEC). f Antibody self-association properties measured by affinity-capture self-interaction nanoparticle spectroscopy (AC-SINS) assay. Data are from one experiment representative of three experiments using three individual donors (a–d). Errors in all plots and tables represent standard deviations from triplicate experiments