Fig. 1. Possible common synaptic and molecular mechanisms underlying L-DOPA-induced and dystonia.
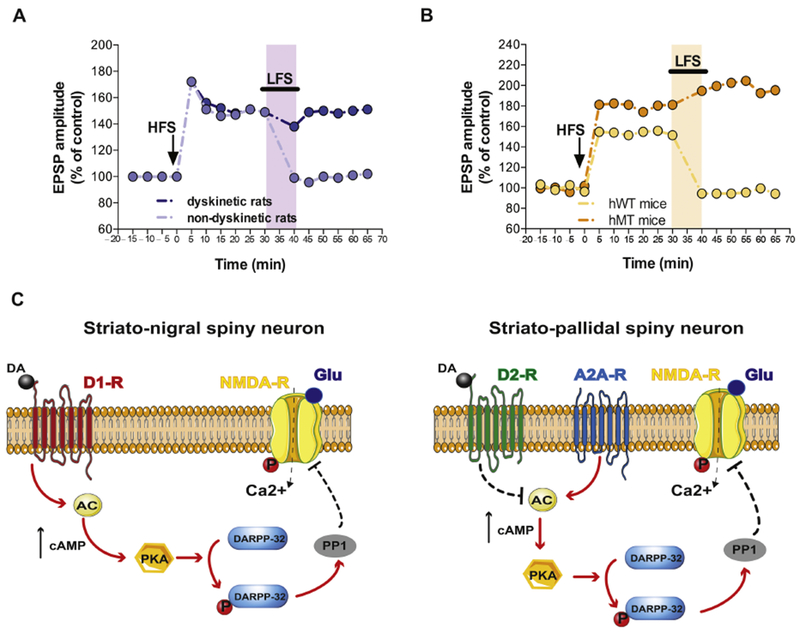
A) Time course of striatal synaptic plasticity (long term potentiation, LTP, and depotentiation) following HFS and LFS protocols in dyskinetic and non-dyskinetic 6-OHDA-lesioned rats. Animals showing therapeutic effects of l-DOPA (light violet) display a full recovery of bidirectional synaptic plasticity (modified from Picconi et al., Nat Neurosci 2003). B) Time course of LTP and synaptic depotentiation in transgenic mice overexpressing human mutant (hMT, orange) and wild type torsinA controls (hWT, yellow) a dystonia model (modified from Martella et al., Brain 2009). Shaded areas in light colors represent LFS protocol application. C) Schematic representation of the signaling pathways underlying the synaptic deficits of striato-nigral (D1-positive, left) and striato-pallidal (D2-positive, right) spiny neurons in levodopa-induced dyskinesia hyperkinetic rats and dystonic mice. In particular, the left panel shows that activation of D1 dopamine receptors leads to an increase of cAMP which in turn stimulates PKA favouring DARPP-32 phosphorylation. This latter event activates PP1 and influence NMDA receptor function. The right panel shows a similar downstream pathway that is initiated by the activation of A2 adenosine receptors rather than by D1 receptors. Note the inhibitory effect on this pathway exerted by D2 dopamine receptors.
Abbreviation: AC, adenylate cyclase; DARPP-32, dopamine- and cAMP-regulated phosphoprotein 32 kDa; P, phosphorylation; PP1, Protein Phosphatase 1; PKA, Protein Kinase A; solid arrows, proposed increasing molecular action; dotted arrows, possible blocking molecular action; black arrows, increased levels.
