Figure 1. Transient cellular behavior of αS.
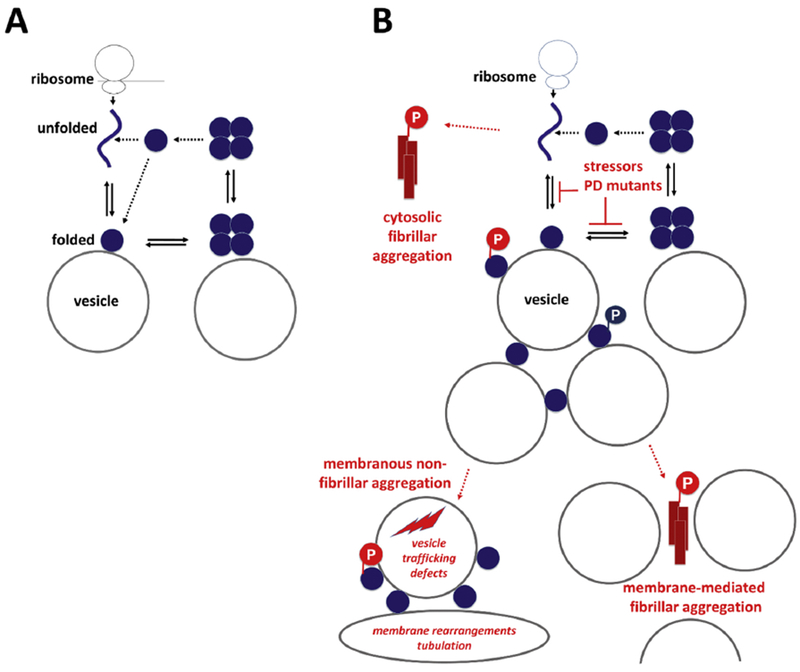
A, Physiological situation: Coming off the ribosome, αS is soluble, unfolded and monomeric. Upon binding to vesicular membranes, it adopts helical fold. Folded monomers transiently assemble to form metastable multimers/tetramers on membranes. Multimers/tetramers are only weakly membrane-associated and likely in an equilibrium with cytosolic multimers/tetramers. Cytosolic tetramers/multimers may have an intrinsic propensity to disassemble - and eventually unfold, initiating a new cycle. B, Pathological situation (pathological states are in red): perturbed cellular αS homeostasis increases i) the levels of aggregation-prone unfolded monomers in the cytosol or ii) the level of membrane-associated monomeric αS. Excess soluble αS monomers may be the starting point of cytosolic fibrillar αS aggregation (top left), while excess membrane association may either cause fibrillar αS aggregation via ‘nucleatin’96 (bottom right) or membranous non-fibrillar αS aggregation (bottom left). The role of phosphorylation atSerl29 is unclear. While primarily considered a pathological event before or in response to fibrillar aggregation (red), there may also be physiological aspects of this post-translational modification (blue).
