Figure 2. Molecular determinants of the transient nature of αS.
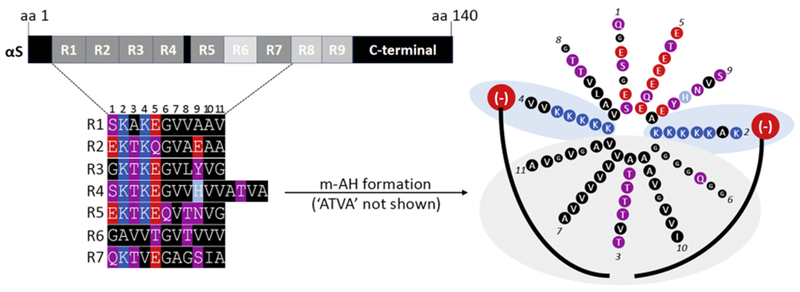
Top left: the 140-aa sequence of human wildtype αS contains 9 semi-conserved 11-aa repeats with the core consensus motif ‘KTKEGV’. Highly conserved repeats are indicated in dark grey, poorly conserved repeats in light grey. Bottom left: color-coded schematic of repeats 1-7 of human αS by aligning the aa sequence via the KTKEGV motifs. Blue indicates basic (light blue: histidine), red: acidic, purple: polar uncharged, and black: non-polar residues. In addition to KTKEGV, the polar character of positions 1 and 9 as well as the non-polar, hydrophobic character of positions 8, 10, and 11 are relatively well conserved. Repeats 1–9 are interrupted only once: by ’ATVA’ between repeat 4 and 5. Right: Color-coded schematic of αS repeats 1-7 (omitting ’ATVA’ between repeats 4 and 5) in an 11/3 helical wheel, embedded in the outer leaflet of a curved vesicle membrane (negatively charged lipid head-groups in red, fatty acid ‘tails’ in black). The helix is stabilized by hydrophobic interactions (gray area) and electrostatic interactions (blue area). Certain structural aspects such as the presence of polar threonines as well as the small and ‘helix-breaking’ glycine prevent the formation of a highly stable helix.
