Highlights
-
•
Reaction time and event-related potentials of inhibitory control were compared in badminton experts and nonathletes.
-
•
Badminton experts showed enhanced inhibitory control and more efficient neural mechanisms.
-
•
Badminton experts performed better inhibitory control processing in re-engagement.
-
•
The re-engagement processing better demonstrated altered brain activity in badminton experts.
Keywords: Badminton athletes, Change-signal task, Event-related potentials, Inhibitory control, Stop-signal task
Abstract
Purpose
The purpose of present study was to investigate the impact of sport experience on response inhibition and response re-engagement in expert badminton athletes during the stop-signal task and change-signal task.
Methods
A total of 19 badminton athletes and 20 nonathletes performed both the stop-signal task and change-signal task. Reaction times (RTs) and event-related potentials were recorded and analyzed.
Results
Behavioral results indicated that badminton athletes responded faster than nonathletes to go stimuli and to change signals, with faster change RTs and change-signal RTs, which take into consideration the variable stimulus onset time mean. During successful change trials in the change-signal task, the amplitudes of the event-related potential components N2 and P3 were smaller for badminton athletes than for nonathletes. Moreover, change-signal RTs and N2 amplitudes as well as change RTs and P3 amplitudes were significantly correlated in badminton athletes. A significant correlation was also found between the amplitude of the event-related potential component N1 and response accuracy to change signals in badminton athletes.
Conclusion
Moderation of brain cortical activity in badminton athletes was more associated with their ability to rapidly inhibit a planned movement and re-engage with a new movement compared with nonathletes. The superior inhibitory control and more efficient neural mechanisms in badminton athletes compared with nonathletes might be a result of badminton athletes’ professional training experience.
Graphical abstract
1. Introduction
Inhibitory control, which encompasses both response inhibition and response re-engagement, plays an essential role in goal-directed behavior in daily life1 as well as in sports. Previous studies have described a positive relationship between sport performance and inhibitory control.2, 3, 4, 5, 6 However, most of these studies focused on response inhibition, which refers to the ability to stop an ongoing or planned action that is no longer appropriate,7, 8, 9 rather than re-engagement to an alternate response. In real life, people are able to withhold ongoing actions when unpredictable events or changes occur, but in the most situations an alternative response has to be executed instead.10, 11 Therefore, response re-engagement may provide another important source of information about the nature of inhibitory control of human executive function.12
In sports, ceasing an action precedes, in most cases, adjusting to an alternative movement. For example, in badminton, a typical reactive sport, players must adjust their movements constantly to an ever-changing environment. Frequently, badminton athletes need to suppress a swing completely, such as when the shuttlecock is outside their range. But more often, they need to inhibit their initial swing first and then switch to a modified one. This response re-engagement reflects cognitive flexibility in inhibitory control,9 which obviously plays a key role in winning a match. Therefore, it is necessary to consider the 2 aspects of inhibitory control to observe the influence of athletic training on human beings. The current study aimed to investigate the effects of badminton expertise on inhibitory control and to explore whether this expert advantage would be revealed in response re-engagement.
Most previous studies that have observed response inhibition in athletes utilized the Go/Nogo task and found that athletes showed higher response accuracy in Nogo trials,13, 14 as well as a faster Go reaction time (RT),14, 15, 16 compared with nonathletes, suggesting superior ability for response inhibition. Event-related potential (ERP) has also been used to further explore the underlying neuronal activity during cognitive processing. In a specific paradigm, several ERP components are elicited by a stimulus. For example, the N2 component, which peaks at approximately 200–350 ms after the target stimuli, always occurs in inhibition tasks and is considered a marker of inhibitory processes.17, 18 Studies of expert baseball athletes and fencers have found a greater amplitude and a shorter latency of N2 in the Nogo portion of Go/Nogo tasks compared with nonathletes,5,14, 15, 16 likely reflecting an improvement of inhibitory control in athletes performing reactive sports. Usually, the P3 component is observed after N2 in the response inhibition task, which occurs approximately 300–500 ms after the target stimuli presentation. In inhibition tasks, the P3 component is believed to reflect the allocation of attentional resources during the inhibitory processing,19 but other studies have posited that it reflected aspects of active inhibition of the motor response.20 However, when P3 amplitude has been compared between athletes and nonathletes, the results have been inconsistent. For example, some studies have found that the P3 amplitude was larger in athletes than in nonathletes during Nogo trials. The studies on fencing have suggested that, because of the higher requirement of high-speed responses to target stimuli (e.g., when a Nogo stimulus is detected), the more cognitive resources was took during the inhibitory processing.15, 16 This hypothesis is supported by the increased P3 amplitude observed in fencers. In contrast, Zhang et al.14 found a reduced P3 amplitude during Nogo trials in experienced fencers compared with nonathletes and suggest that this reduced Nogo-P3 in fencers reflects their improved inhibitory function, which allows their brains to evaluate and monitor response inhibition more efficiently.
In addition to these 2 classic components discussed, the early and exogenous component N1 is also an important component in inhibitory tasks. The N1 component reflects the attention paid to the target stimuli.21 An enhanced N1 amplitude, indicative of early visual processing, has also been observed among athletes during response inhibition.15, 16 Using the stop-signal task (SST), researchers have reported that the amplitude of N1 in successfully stopped trials is larger than that in unsuccessfully stopped trials, suggesting a positive association between N1 amplitude and response inhibition.21 Together with N2 and P3, the N1 component in athletes needs further consideration in the SST. In the present study, we aim to describe the relationship between early visual processing (reflected by the N1 amplitude) and response inhibition (reflected by N2 and P3) in expert athletes compared with nonathletes.
On the basis of these studies, it seems that athletes engaged in reactive sports have developed superior response inhibition, as reflected by their correspondingly altered neural activities, although some results remain unclear. However, little focus has been given to response re-engagement, and further studies are needed to get a complete understanding of the effects of expert advantage on inhibitory control. The change-signal task (CST) was designed to investigate response re-engagement in inhibitory control.9 The latency of re-engagement can be measured by change RT; the latency of inhibition can also be determined by calculating the change-signal RT (CSRT). Thus, the 2 components of inhibitory control—response inhibition and re-engagement—can be measured and assessed using the SST and CST, respectively.
The SST and the Go/Nogo are 2 popular tasks that can be used to assess response inhibition, but studies have shown that these 2 tasks are not completely identical measures of response inhibition.22 In the Go/Nogo task, the response is stimulus dependent, which requires a response selection between executing and inhibiting a motor response. That is, a motor response is made to Go stimuli and withheld to Nogo stimuli. In SST and CST, responses are made on every trial unless a stop or a change signal is presented, and the inhibitory processing is triggered by a stop/change signal following the Go signal. This means that subjects have to retract an executive response that has already been triggered by the Go signal to successfully stop.23 Therefore, we believe that the response processes of SST are more relevant to actual sports scenarios than are those of the Go/Nogo task, even though the latter has been more frequently studied in athletes. Furthermore, the 2 classic components N2 and P3, which are elicited by the Nogo stimuli and stop signal, have different explanations in these 2 tasks. For example, Nogo-N2 reflects the inhibitory processes for this task,24, 25 whereas other studies have suggested that the stop-N2 does not reflect response inhibition but instead reflects evaluation of the stop signal.20, 26 Nogo-P3 reflects the attentional processing involved in stimulus evaluation,27 and stop-P3 represents the cognitive control engagement in monitoring outcome during the inhibitory process.28, 29 Because most studies have investigated the response inhibition in athletes using the Go/Nogo task, it is necessary to apply SST and CST to further explore the advantages of response inhibition and response re-engagement in reactive athletes, and thus make a contribution to our knowledge of the comparison of Go/Nogo and SST.
In the present study, we aimed to investigate the effects of response inhibition and re-engagement in 2 tasks of inhibitory control in badminton athletes compared with nonathletes. We anticipated that, as an effect of long-term badminton training, badminton athletes would show enhanced inhibition and re-engagement relative to nonathletes. In their ERP components, we expected that the typical inhibitory control-related components N2 and P3 would show significant differences between badminton athletes and nonathletes. In addition, we expected that there would be increased effort in perceptual processing, as reflected by the N1 component in badminton athletes compared with nonathletes. Finally, on the basis of previous studies that found correlations between inhibition performance and ERP components,5 we hypothesized that a similar correlation would be found among the participants in our study.
2. Methods
2.1. Participants
We conducted the power analysis before the study, for a 2 (number of groups) × 2 (number of measurements) repeated measures design, the total sample size was determined using G*power30 with the expected effect size of 0.4 and a significance level of 0.05 at the desired power of 0.95. For the athletes group, 19 badminton athletes (5 women aged 19.03 ± 0.71 years, and 14 men aged 20.01 ± 1.20 years, mean ± SD) were recruited from the Shanghai University of Sport badminton team. The inclusion criteria included (1) having more than 5 years of professional training (>12 h/week) before being placed on the college team, (2) maintaining skill training (>5 h/week) during college, and (3) these athletes are above the 2nd level of the national standard.
For the nonathletes group, 20 college students (6 women aged 18.24 ± 0.42 years and 14 men aged 19.42 ± 1.91 years) were recruited from the Shanghai University of Sport. The inclusion criteria included (1) with no sports training experience, and (2) watch the badminton matches less than 5 times in all during the past 5 years. The research was approved by the Research Ethics Committee at Shanghai University of Sport (approval number 2015003).
All participants completed a personal information table before they started the experiment. All participants were undergraduates who did not smoke. They all had normal or corrected-to-normal visual acuity and were right handed. None reported mental or organic diseases. All participants provided written informed consent before inclusion in this study and were financially compensated for their participation.
2.2. Task procedures
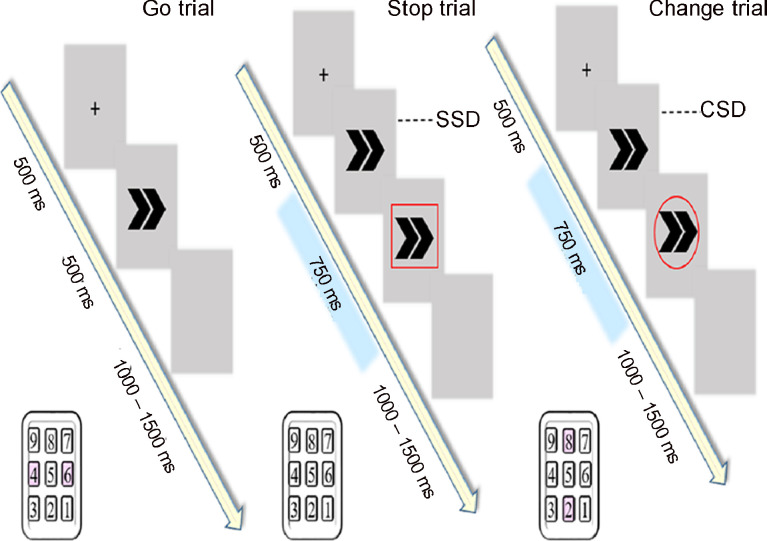
The participants were provided with instructions for the 2 tasks: the SST and the CST—and the sequence counterbalance was used. In SST, all trials started with a 500-ms presentation of a cross on a screen. The participants were instructed to press number keys on the numeric keypad with their right index finger, resting on but not pressing “5” before and after each trial. The Go stimulus, an arrow pointing to the left or right (50% each), was presented after the cross disappeared. Participants were instructed to press the “4” key when the arrow pointed to the left and the “6” key when the arrow pointed to the right. Participants were told to respond as quickly as possible. The arrow disappeared from the screen if no key was pressed within 500 ms (Fig. 1, left). There was a 30% chance in each block of trials that the stop signal for the SST (a red square outline) would appear surrounding the arrow. The appearance of the stop signal in the SST indicated that the participants had to withhold all key presses until the next trial (Fig. 1, center). A blank screen appeared between each trial for a randomly generated time ranging between 1000 ms and 1500 ms.
Fig. 1.
Illustration of the 3 types of trials: go (left), stop (center), and change (right). The SST consists of go trials and stop trials, whereas the CST consists of go trials and change trials. Stimulus onset (SSD for SST; CSD for CST) fluctuates between 50 ms and 700 ms. Numbers with gray background indicate which keys are to be pressed for each task. CST = change-signal task; CSD = change-signal delay; SSD = stop-signal delay; SST = stop-signal task.
In the CST, the procedures were much the same as for the SST. The trials consisted of the Go stimulus and change signals. Participants were instructed to press the “4” key and the “6” key according to the orientation of the arrow (Fig. 1, left). There was a 30% chance in each block of trials that the change signal for the CST (a red circle outline) would appear surrounding the arrow. The appearance of the change signal in the CST indicated that the participants should switch target keys, such that arrows pointing to the left now required pressing “8” and those pointing to the right required pressing “2” (Fig. 1, right).
The delay between a Go stimulus and a stop signal or a change signal fluctuated (stimulus onset asynchrony) from 50 ms to 700 ms; the delay was determined by a staircase-tracking algorithm that adapted to the response rate.31, 32 The original delay in each block was 200 ms, and when participants successfully stopped a stop signal trial in the SST or successfully changed their response in the change signal trial in the CST, the stimulus onset would increase by 50 ms in the next stop or change signal trial to increase the task difficulty. Otherwise, the stimulus onset was decreased by 50 ms to decrease the difficulty. This tracking procedure ensured an approximately 50% success rate in the stop or change signal trials.
The experiment was conducted in a dimly lit, sound-attenuated room. Participants were seated 100 cm in front of a screen, which was positioned at eye level. All participants were required to read the instructions before they began. They conducted 20 trials of each task for practice; these results were not included in the analyses. In the formal experiment, there were 3 blocks for the SST and 3 blocks for the CST, with each block containing 120 trials comprising 84 go trials and 36 stop trials for the SST or change trials for the CST. The sequence in which the participants performed the 2 tasks was counterbalanced across participants. All tasks were programmed using Matlab (The MathWorks Inc., Natick, MA, USA), and stimuli were presented on a 19-inch cathode ray tube screen (1024 × 768 pixels; refresh rate: 100 Hz).
2.3. Data collection
The behavioral response data were collected using Matlab software (R2013b; The MathWorks Inc.). Meanwhile, the electroencephalographic (EEG) data were recorded using a Brain Vision EEG system (Brain Products GmbH, Gilching, Germany) referenced against FCz with a 64-channel amplifier and a sampling frequency of 500 Hz. Continuous EEG measurements were taken, averaged from the right and left mastoids, and the ground electrode was located on the mid-forehead. Horizontal eye movement blinks and vertical eye movements were recorded. The electrode impedances were maintained below 10 kΩ for all electrooculogram and EEG electrodes.
2.4. Data and statistical analyses
2.4.1. Behavioral data
Four dependent variables were calculated for each task and group. First, response accuracy was calculated for go trials and stop trials in the SST, and for the go trials and change trials in the CST. Second, the go RT of go trials in 2 tasks was determined by measuring the time interval between the time when go signal appeared and time when participants made the correct response. Third, the signal RT (stop-signal RT (SSRT), CSRT) was calculated based on the mean stimulus onset (stop/change-signal delay (SSD, CSD)) in correct signal trials and the mean go RT for both the SST and CST using the following equations: SSRT = mean go RT – mean SSD; CSRT = mean go RT – mean CSD. Fourth, the re-engagement RT was averaged for the correct responses. Specifically, the re-engagement RT was recognized as the change RT, which was calculated as the time duration between the onset of the change signals and the participants’ correct key pressing (the dependent variable table is presented in Supplementary Table 1).
The Monte Carol simulations of Band et al.33 showed that the integration method resulted in reliable SSRT estimates for central SSDs, that is, SSDs for which p (respond|signal) is close to 0.50. Therefore, we reanalyzed the data using the integration method, that is, subtracting the mean SSD/CSD from the nth RT, where the nth RT is determined by multiplying the number of RTs in the go RT distribution by the overall p (respond|signal).
Furthermore, dependent variables for errors and stop/change-signal delays were calculated for each task and group. (The table of variables for errors and signal delays is available in Supplementary Table 2.)
Two-way repeated measures analysis of variance (ANOVA) was performed for go accuracy, inhibition (stop/change) accuracy, go RT, and signal RT (SSRT/CSRT), with group (badminton athletes vs. nonathletes) as the between-subjects factor and the inhibitory task (SST vs. CST) as the within-subjects factor. For change RT, variables for errors, and stop/change-signal delays, independent-samples t tests were used between groups.
2.4.2. ERP data
The EEG data were analyzed using EEGLAB toolbox34 in Matlab R2013b. All channels were re-referenced offline to the average of the 2 mastoid electrodes. Eye movement and blinks were rejected for ocular correction. The recorded EEG data were filtered with a digital bandpass filter set from 0.1 Hz to 30.0 Hz to reduce low-frequency content that was irrelevant to the components of interest. Additionally, a notch filter (50 Hz) was applied to the data. The EEG data were then segmented with respect to stimulus markers into 1900-ms epochs, with 1100 ms before and 800 ms after the onset of the stop or change stimulus. Only trials that were correctly responded to were included in analyses. Trials with amplitudes exceeding ±100 µV were excluded. To baseline correct to stable brain activity, the mean amplitude of the 900- to 1100-ms (blank screen) prestimulus interval was selected as the baseline, because the go stimulus was always before the signal stimulus and the ERP elicited by the go stimulus would be overlapped with the prestimulus interval if this were selected as the baseline. The ERP data were averaged for each group and condition. Finally, fast Fourier transformation35 was used to filter the ERPs.
Using the grand average waveforms and scalp topographic distributions, we analyzed 3 time windows: 120–180 ms for N1, 160–220 ms for N2, and 300–400 ms for P3 (The figures for waveforms are available online as Supplementary Fig. 1 for SST and Supplementary Fig. 2 for CST). For the N1 component, 2 electrode sites were grouped in the left hemisphere (O1, PO7) and 2 in the right hemisphere (O2, PO8). A two-way repeated measures ANOVA was used to examine N1 amplitudes, with group (badminton athletes vs. nonathletes) as the between-subjects factor and hemisphere (left vs. right) as the within-subjects factor. For the N2 and P3 components, according to our topographic distributions and previous studies,19, 28 we selected 4 midline electrodes (Fz, FCz, Cz, and Pz) for further analysis, and the amplitudes were entered into a two-way repeated measures ANOVA with group (badminton athletes vs. nonathletes) as the between-subject factor, electrode sites (Fz, FCz, Cz, and Pz) as the within-subjects factor. (The peak amplitudes and latencies of N1, N2, and P3 components are available online as Supplementary Data, the Result section.)
To investigate associations between cognitive processing and inhibitory control ability, we analyzed the following bivariate correlations: stop/change accuracy and N1 amplitude at the occipital electrodes (O1, O2, PO7, and PO8). We performed the K-S test on some data that are used in Pearson's correlations between 2 groups (SSRT, CSRT, stop ACC, change ACC, change RT, and the amplitudes of N1, N2, and P3). The results show that the distribution of these dependent variables is normal in controls (all p > 0.1) and in badminton athletes (all p > 0.1, except the stop ACC, p = 0.036). Considering the abnormal distribution of the stop accuracy in badminton athletes, we did an outlier analysis and took out 2 outliers. The K-S test shows the normal distribution of stop ACC (badminton athletes) after taking out the 2 outliers (Z = 0.772, p = 0.591). Pearson's correlation was then performed for SSRT/CSRT and N2 amplitude and for N1 and P3 amplitude in CST.
For all analyses (behavioral and EEG data), p values of less than 0.05 were statistically significant. The p value ranges from 0.05 to 0.08 were marginally significant. (Degrees of freedom were corrected using the Greenhouse–Geisser method.) The effect size for each comparison are reported, and the partial eta-squared () was used as an index of effect size. Post hoc tests of significant main effects were conducted using the least significant difference method. The test was highly sensitive, and slight differences in the mean value of each level might be detected.
3. Results
3.1. Behavioral results
The mean go RT, SSRT, CSRT, and change RT were included in the statistical analyses. The stop rate and change rate of both the experts and nonathletes were close to the expected 50% (Table 1).
Table 1.
Behavioral data associated with the 2 tasks.
| Measurements | Task | Variables | Badminton athletes | Nonathletes | Cohen's ds |
|---|---|---|---|---|---|
| (n = 19) | (n = 20) | ||||
| Accuracy (%) | SST | Go-ACC | 98.45 ± 2.09 | 97.78 ± 1.98 | 0.33 |
| Stop-ACC | 47.81 ± 3.80 | 49.44 ± 1.14 | 0.58 | ||
| CST | Go-ACC | 98.04 ± 3.24 | 98.10 ± 1.37 | 0.02 | |
| Change-ACC | 47.03 ± 5.63 | 45.51 ± 6.30 | 0.25 | ||
| RT (ms) | SST | Go-RT | 424.01 ± 55.59 | 460.39 ± 50.39 | 0.69 |
| CST | Go-RT | 432.67 ± 60.48 | 450.04 ± 38.67 | 0.35 | |
| Change-RT | 654.80 ± 69.96 | 724.52 ± 88.60* | 0.87 | ||
| Signal RT (ms) | SST | SSRT | 282.84 ± 17.26 | 288.60 ± 29.67# | 0.24 |
| CST | CSRT | 287.22 ± 18.16 | 309.22 ± 43.45* | 0.66 |
Notes: The effect sizes are calculated by Cohen's ds. Values are mean ± SD.
Abbreviations: ACC = accuracy; CSRT = change signal reaction time; CST = change-signal task; RT = reaction time; SSRT = stop-signal reaction time; SST = stop-signal task.
p < 0.05, significantly different compared with badminton athletes;
p < 0.001 significantly different compared with CSRT in nonathletes.
For the go accuracy, the main effect of task (F (1, 37) = 0.031, p = 0.861, = 0.001) and group (F (1, 37) = 0.357, p = 0.554, = 0.010) were not significant. The interaction between task and group was not significant (F (1, 37) = 0.559, p = 0.459, = 0.015).
For the inhibition accuracy, the main effect of task (F (1, 37) = 9.144, p = 0.005, = 0.198) was significant, indicating that the stop accuracy (48.63%) was higher than the change accuracy (46.27%) and the interaction between task and group was significant (F (1, 37) = 4.096, p = 0.050, = 0.100). Further analyses revealed that the stop accuracy in badminton athletes was slightly lower than in nonathletes (p = 0.073). The main effect of group was not significant (F (1, 37) = 0.002, p = 0.963, < 0.001).
For the RT in the go trials of both tasks, the main effect of group was marginally significant (F (1, 37) = 3.362, p = 0.075, = 0.083), indicating that the go RT in badminton athletes (428.34 ± 57.46 ms) was shorter than that in nonathletes (455.22 ± 44.64 ms). The main effect of task (F (1, 37) = 0.012, p = 0.913, < 0.001) and the interaction between task and group (F (1, 37) = 1.508, p = 0.227, = 0.039) were not significant.
By contrast, for the signal RT, the main effect of task (F (1, 37) = 12.127, p = 0.001, = 0.248) and the interaction between task and group were significant (F (1, 37) = 6.953, p = 0.012, = 0.158). Further analyses revealed that the badminton athletes had a significantly shorter CSRT than nonathletes did (p = 0.046) and that the CSRT was significantly longer than the SSRT in nonathletes (p < 0.001). The comparisons of the mean signal RTs and change RT are available Supplementary Fig. 3. The main effect of group was not significant (F (1, 37) = 2.146, p = 0.151, = 0.055).
For the change RT, an independent-samples t test revealed that the change RT in badminton athletes was significantly shorter than in nonathletes (t (37) = 2.718, p = 0.01, Cohen's d = 0.873).
For the error-related variables and stop/change-signal delay, an independent-samples t test revealed that there were no significant differences between 2 groups except the go-error in SST. The table of behavioral data for errors is available in Supplementary Table 3.
3.2. ERP Results
3.2.1. Stop-signal ERPs
No significant differences in amplitude were found for the N1 component (150–180 ms).
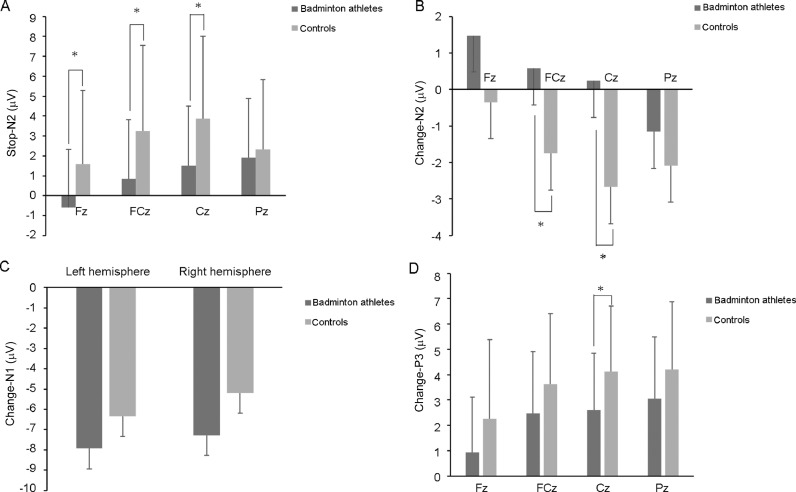
For the N2 component (160–220 ms) amplitude, the main effect of group was significant (F (1, 37) = 4.227, p = 0.047, = 0.103), with the badminton athletes (–0.92 ± 0.67 μV) displaying a smaller amplitude than the nonathletes (–2.84 ± 0.65 μV). The main effect of electrode site was also significant (F (2, 61) = 7.674, p < 0.001, = 0.172), with the smallest N2 amplitude on Fz (–0.55 ± 0.53 μV) (all p < 0.002; FCz: −2.08 ± 0.59 μV, Cz: −2.72 ± 0.57 μV, Pz: −2.17 ± 0.52 μV). The interaction of electrode site by group was not significant (F (2, 64) = 1.933, p = 0.160, = 0.050) (Fig. 2A).
Fig. 2.
Comparison of the amplitudes at various electrode sites between badminton athletes and nonathletes for each task. (A) The N2 amplitude at 4 electrode sites in participants performing the SST. (B) The N2 amplitude at 4 electrode sites in participants performing the CST. (C) The N1 amplitudes for both hemispheres in participants performing the CST. (D) The P3 amplitudes at 4 electrode sites in participants performing the CST. * p < 0.05 significant difference. CST = change-signal task; SST = stop-signal task.
No significant differences were found in the amplitude of the P3 component (300–400 ms).
3.2.2. Change-signal ERPs
For the amplitude of the N2 component (160–220 ms), the main effect of group was significant (F (1, 37) = 4.770, p = 0.035, = 0.114), with the badminton athletes (0.28 ± 0.66 μV) displaying a smaller amplitude than the nonathletes (–1.72 ± 0.64 μV). The main effect of electrode site was also significant (F (1, 55) = 7.066, p = 0.004, = 0.160), with the smallest N2 amplitude on Fz (−0.55 ± 0.51 μV) (all p < 0.004; FCz: −0.59 ± 0.53 μV, Cz: −1.21 ± 0.57 μV, Pz: −1.63 ± 0.61 μV). The interaction of electrode site by group was not significant (F (1, 55) = 1.358, p = 0.261, = 0.035) (Fig. 2B).
For the amplitude of the N1 component, the main effect of hemisphere was significant (F (1, 37) = 7.467, p = 0.010, = 0.168), with the N1 amplitude in the left hemisphere (−7.11 ± 0.62 μV) larger than that in the right hemisphere (−6.20 ± 0.53 μV). The main effect of group on the average amplitude was not significant (F (1, 37) = 2.847, p = 0.100, = 0.071) nor was the interaction of hemisphere and group (F (1, 37) = 0.827, p = 0.369, = 0.022) (Fig. 2C).
For the amplitude of the P3 component (300–400 ms), the main effect of group was marginally significant (F (1, 37) = 3.833, p = 0.058, = 0.094), with the badminton athletes (2.26 ± 0.50 μV) displaying a smaller amplitude than the nonathletes (3.64 ± 0.49 μV). The main effect of electrode site was also significant (F (1, 48) = 13.928, p < 0.001, = 0.273), with the smallest P3 amplitude on Fz (1.63 ± 0.43 μV) (all p < 0.004; FCz: 3.08 ± 0.42 μV, Cz: 3.42 ± 0.39 μV, Pz: 3.67 ± 0.41 μV). The interaction of electrode site by group was not significant (F (1, 48) = 0.097, p = 0.825, = 0.003) (Fig. 2D).
3.3. Correlation between EEG and behavioral data
3.3.1. N1 amplitude and accuracy
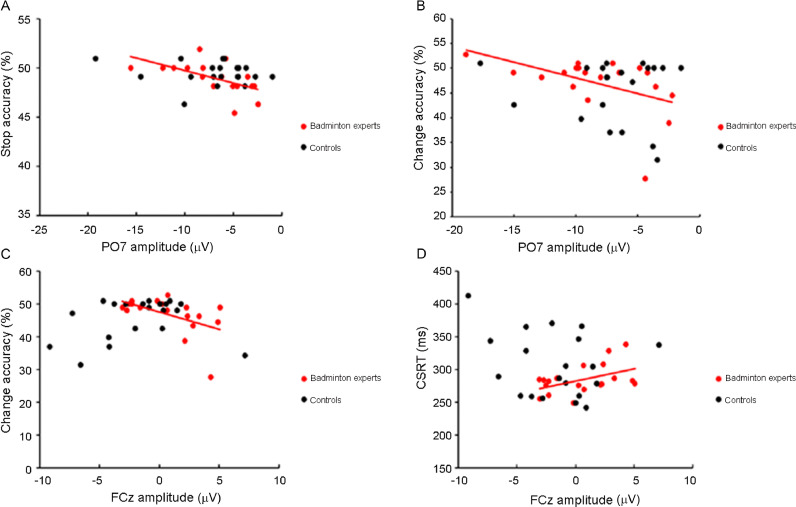
In SST, a correlation analysis of badminton athletes revealed a significant negative correlation between accuracy in the change trials and the N1 amplitude on the PO7 electrode site (p = 0.015, r = –0.578), as well as that in CST (p = 0.036, r = –0.483). However, the correlation of nonathletes was not significant (p = 0.731, r = –0.082) (Fig. 3A, B).
Fig. 3.
Correlations between behavioral and ERP data. (A) Correlation between the N1 component amplitude at the PO7 electrode site and the accuracy of badminton athletes and nonathletes during performance of a stop trial. (B) Correlation between N1 amplitude at the PO7 electrode site and the accuracy of badminton athletes and of nonathletes during performance of a change trial (C) Correlation between N2 amplitude at the FCz electrode site and the accuracy of badminton athletes and nonathletes during performance of a change trial. (D) Correlation between N2 amplitude at the FCz electrode site during performance of the CSRT of badminton athletes and of nonathletes. CSRT = change-signal RT; ERP = event-related potential.
3.3.2. N2 amplitude and accuracy, RT
In the CST, a correlation analysis of badminton athletes revealed negative correlations between the change ACC and the N2 amplitude at the FCz (p = 0.021, r = –0.523) and Cz (p = 0.001, r = –0.698) electrode sites. These same correlations in the nonathletes were not significant (FCz: p = 0.374, r = −0.210; Cz: p = 0.254, r = 0.268) (Fig. 3C).
Correlation analyses of badminton athletes revealed positive correlations between the CSRT and the N2 amplitude at the FCz (p = 0.045, r = 0.464) and Cz (p = 0.042, r = 0.471) electrode sites. However, the correlation of nonathletes was not significant (FCz: p = 0.294, r = −0.247; Cz: p = 0.326, r = −0.231) (Fig. 3D).
3.3.3. N1 amplitude during change trials and P3 amplitude
In CST, there was a significant negative correlation between N1 amplitude (left hemisphere) and P3 amplitude at the Fz (p = 0.052, r = –0.452), Cz (p = 0.061, r = –0.438) and Pz (p < 0.001, r = –0.740). However, the correlation of nonathletes was not significant (Fz: p = 0.469, r = 0.172; Cz: p = 0.103, r = –0.385; Pz: p = 0.134, r = –0.347). The figure showing this correlation is available in Supplementary Fig. 4.
4. Discussion
In this study, we investigated response inhibition and re-engagement of expert badminton athletes compared with nonathletes. It is the first time that these 2 aspects of inhibitory control have been investigated among athletes in 1 study. The present study illustrates the cognitive superiority of badminton athletes and sheds new light on the impact of athletic training on inhibitory control. Subjects were asked to inhibit a prepared response completely or to switch actions upon the appearance of a stop signal (in SST) or a change signal (in CST). Behaviorally, athletes showed shorter response inhibition and re-engagement times than nonathletes. Electrophysiologically, we found that, compared with nonathletes, experts showed reduced N2 amplitudes during stop trials, and reduced N2 and P3 amplitudes during change trials. Significant correlations between CST behavioral performance and ERPs were found in the badminton athletes but not in the nonathletes.
The present behavioral results are consistent with the hypothesis that badminton athletes would exhibit a shorter CSRT than nonathletes would. According to the race model,32 response inhibition depends on competition between the processes of response execution and inhibition, and a successful inhibition requires the latter process to “win the race”. Thus, a shorter CSRT indicated that badminton athletes were faster at inhibiting an initiated movement than were nonathletes. Moreover, as previously described, the shorter response time of badminton athletes in go trials further support the notion of enhanced inhibition, namely, experts can successfully cease the action even though this action was too fast. We found that experts also had shorter CSRTs than nonathletes in the CST; that is, badminton athletes were faster than nonathletes at switching to a new action. Together, these results suggest that badminton athletes outperform nonathletes in inhibitory control, especially when it comes to response re-engagement processing.
However, not all of our results were consistent with previous studies; we did not observe a significant difference between groups in response inhibition as assessed by the SST.36 Considering the faster go response time and lower Nogo accuracy in badminton athletes (described previously), we hypothesized that badminton athletes would take the go trial as a priority, resulting in a response that could not be stopped immediately and quickly. However, in the CST, they seemed to prioritize the change trial and response to go trial equally, allowing them to keep the same inhibition time as in the SST, despite the increased cognitive load with CST.9 For nonathletes, the longer response time in the CST than in the SST might be due to the larger cognitive load associated with the CST, in which participants need to complete 2 successive cognitive processes. Accordingly, we argue that cognitive processing in the CST fits the actual processing of badminton athletes during their real-world sports experience better than it does in the SST. Measures obtained in the relatively simpler SST did not distinguish badminton athletes from nonathletes, indicating an advantage of examining inhibitory control processing in athletes using the CST rather than the SST.
Our EEG results also showed differences between experts and nonathletes, primarily on the CST. Compared with nonathletes, experts showed reduced amplitudes of both the stop-N2 (the N2 component elicited by stop trials) and the change-N2 (the N2 component elicited by change trials). Considering that EEG studies on the re-engagement processes in athletes are lacking, we will cite studies on nonathletes to discuss these results. Studies have shown similar neurophysiological activity during both stopping and changing of a motor response.1, 37 Thus, we believe that the change-N2 amplitude may reflect that of the stop-N2. However, interpretation of N2 component results across different inhibition processing tasks remains controversial. Nieuwenhuis et al.38 argued that the N2 component reflects the conflict arising from competition between the execution and the inhibition of a single response; this interpretation suggests that the conflict is smaller in badminton athletes than in nonathletes because the inhibitory response overrode the go response much more quickly. In addition, the positive correlation we found between change-N2 amplitude and CSRT in badminton athletes also supported this interpretation of N2 amplitude, suggesting that badminton athletes stop faster because of the reduced conflict, and thus reduced workload, in their brains during task performance. Taken together, these findings indicate that badminton athletes are better than nonathletes at stopping a prepared response in both tasks because of reduced cognitive load. Notably, this result is in contrast to most previous studies examining the N2 amplitude in highly trained athletes performing a Go/Nogo paradigm, which instead show an increased stop-N2 amplitude. This indicates that processing of stop/change signals in the SST/CST cannot be simply equated with processing of Nogo stimuli in the standard Go/Nogo task.
Badminton athletes also displayed reduced P3 amplitudes elicited by change signals compared with nonathletes. This result is consistent with a previous study among fencers, where a reduced Nogo–P3 was reported, which the authors interpreted as reduced cognitive effort.14 As a late ERP component, P3 reflects the attentional processing involved in stimulus evaluation27 and the cognitive control engagement in monitoring outcome during the inhibitory process.28, 29 The lower P3 amplitude in badminton athletes compared with nonathletes in our study suggests that the experts allocated less attention to the re-engagement response, whereas nonathletes used more cognitive and attentional resources to monitor the results of the inhibition and to ensure the processing of a re-engagement response. In addition, we found a negative correlation between P3 amplitude and N1 in the experts; that is, a smaller P3 amplitude was associated with a larger N1 amplitude. The foregoing correlation result showed that the larger N1 amplitude was associated with the higher change ACC in badminton athletes and suggests that badminton athletes deliberately allocated fewer attentional resources to evaluate and monitor the results of the response inhibition. However, they engaged more resources in the early processing of the change signal to ensure the higher change ACC and the response movement execution, thus making their responses faster than those of the nonathletes. The negative correlation between N1 and P3 amplitude may reflect the efficient resource allocation in badminton athletes. The reduced P3 amplitude was detected only during the change task, not the stop task, providing further evidence that the training experience in reactive sports is more similar to the demands of the CST than to the demands of the SST.
We unexpectedly observed that there was no difference between experts and nonathletes in the change-N1 amplitude, suggesting that early visual processing of change signals is not altered as a function of athletic experience. However, we did find a significant negative correlation between change-N1 amplitude and change accuracy in badminton athletes, but not in nonathletes. Several studies have described a positive relationship between early attentional processing and inhibitory control.21,39, 40, 41 Thus, our results suggest that for badminton athletes successful change was related to attentional effort on the change signal. We agree with that this finding indicates a kind of specialized processing in experts’ brains described by Callan and Naito42 which posited “a specific brain region (or network of regions) carrying out processes related to aspects of a task through experience-dependent learning, thus allowing for better performance” (p. 2). Further study will be required to investigate the early attentional processing involved in performance of inhibitory control tasks in badminton athletes. This would help to optimize the training program and the selection of badminton athletes.
The present findings highlight the superior inhibitory control of athletes compared with nonathletes when undertaking 2 different tasks. However, this study had some limitations that should be considered. Although we speculate that our findings might be broadly relevant to long-term reactive sports training, the generalizability of these results across different activities remains to be determined. And the insufficient sample size prevents us from coming up with a stronger conclusion. Furthermore, it is widely understood that a strong relationship exists between physical fitness and cognitive function. Thus, future studies should take into account the physical fitness of participants,43 for example, by determining body mass index and maximum oxygen uptake, to elucidate the specificity of reactive sports training on inhibitory control processes.
5. Conclusion
In the present study we observed superior inhibitory control in badminton athletes, who inhibited their responses and reengaged alternative movements more quickly than did the nonathletes. Measures of brain activity in badminton athletes suggested that they were more efficient at stopping a prepared response owing to their allocation of fewer cognitive resources to updating the movement. This advantage could indicate a more rational distribution of neural resources in badminton athletes, which would allow them to achieve better and more stable performance than nonathletes. The efficient neural mechanisms and superior inhibitory control observed in badminton athletes may be a direct result of their long-term professional training.
Acknowledgments
Acknowledgment
This research received specific grants from the Natural Science Foundation of China (31571151, 31700985) and the Scientific and Technological Commission of Shanghai (17080503100).
Authors' contributions
JC designed and carried out these experiments, and drafted the manuscript; GZ participated in the EEG data analysis; YL and XJ participated in the coordination of the study and helped polish the manuscript; YL and CZ conceptualized the study, revised/reviewed the manuscript, and provided the financial support for this research. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2019.05.005.
Contributor Information
Yingzhi Lu, Email: yingzhi.lu.sus@gmail.com.
Chenglin Zhou, Email: chenglin_600@126.com.
Appendix. Supplementary materials
References
- 1.Rangel-Gomez M., Knight R.T., Krämer U.M. How to stop or change a motor response: laplacian and independent component analysis approach. Int J Psychophysiol. 2015;97:233–244. doi: 10.1016/j.ijpsycho.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brevers D., Dubuisson E., Dejonghe F., Dutrieux J., Petieau M., Cheron G. Proactive and reactive motor inhibition in top athletes versus non-athletes. Percept Mot Skills. 2018;125:289–312. doi: 10.1177/0031512517751751. [DOI] [PubMed] [Google Scholar]
- 3.Liao K.F., Meng F.W., Chen Y.L. The relationship between action inhibition and athletic performance in elite badminton players and non-athletes. J Hum Sport Exerc. 2017;12:574–581. [Google Scholar]
- 4.Krenn B., Finkenzeller T., Würth S., Amesberger G. Sport type determines differences in executive functions in elite athletes. Psychol Sport Exerc. 2018;38:72–79. [Google Scholar]
- 5.Yamashiro K., Sato D., Onishi H., Sugawara K., Nakazawa S., Shimojo H. Skill-specific changes in somatosensory Nogo potentials in baseball players. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang E.C., Chu C.H., Karageorghis C.I., Wang C.C., Tsai J.H., Wang Y.S. Relationship between mode of sport training and general cognitive performance. J Sport Health Sci. 2017;6:89–95. doi: 10.1016/j.jshs.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logan G.D. On the ability to inhibit thought and action: a users' guide to the stop signal paradigm. In: Dagenbach D., Carr T.H., editors. Inhibitory processes in attention, memory, and language. Academic Press; San Diego, CA: 1994. pp. 189–239. [Google Scholar]
- 8.De Jong R., Coles M.G., Logan G.D. Strategies and mechanisms in nonselective and selective inhibitory motor control. J Exp Psychol Hum Percept Perform. 1995;21:498–511. doi: 10.1037//0096-1523.21.3.498. [DOI] [PubMed] [Google Scholar]
- 9.Boecker M., Gauggel S., Drueke B. Stop or stop-change–does it make any difference for the inhibition process? Int J Psychophysiol. 2013;87:234–243. doi: 10.1016/j.ijpsycho.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Krämer U.M., Knight R.T., Münte T.F. Electrophysiological evidence for different inhibitory mechanisms when stopping or changing a planned response. J Cogn Neurosci. 2011;23:2481–2493. doi: 10.1162/jocn.2010.21573. [DOI] [PubMed] [Google Scholar]
- 11.Scanlan A.T., Fox J.L., Borges N.R., Tucker P.S., Dalbo V.J. Temporal changes in physiological and performance responses across game-specific simulated basketball activity. J Sport Health Sci. 2018;7:176–182. doi: 10.1016/j.jshs.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Jong R., Coles M.G., Logan G.D. Strategies and mechanisms in nonselective and selective inhibitory motor control. J Exp Psychol Hum Percept Perform. 1995;21:498–511. doi: 10.1037//0096-1523.21.3.498. [DOI] [PubMed] [Google Scholar]
- 13.Chan S.Y., Wong A.C.N., Liu Y., Yu J.J., Yan J.H. Fencing expertise and physical fitness enhance action inhibition. Psychol Sport Exerc. 2011;12:509–514. [Google Scholar]
- 14.Zhang D., Ding H., Wang X., Qi C., Luo Y. Enhanced response inhibition in experienced fencers. Sci Rep. 2015;5:16282. doi: 10.1038/srep16282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Russo F., Taddei F., Apnile T., Spinelli D. Neural correlates of fast stimulus discrimination and response selection in top-level fencers. Neurosci Lett. 2006;408:113–118. doi: 10.1016/j.neulet.2006.08.085. [DOI] [PubMed] [Google Scholar]
- 16.Taddei F., Bultrini A., Spinelli D., Di Russo F. Neural correlates of attentional and executive processing in middle-age fencers. Med Sci Sports Exerc. 2012;44:1057–1066. doi: 10.1249/MSS.0b013e31824529c2. [DOI] [PubMed] [Google Scholar]
- 17.Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biol Psychol. 1993;35:123–138. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- 18.Schmajuk M., Liotti M., Busse L., Woldorff M.G. Electrophysiological activity underlying inhibitory control processes in normal adults. Neuropsychologia. 2006;44:384–395. doi: 10.1016/j.neuropsychologia.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Chu C.H., Alderman B.L., Wei G.X., Chang Y.K. Effects of acute aerobic exercise on motor response inhibition: an ERP study using the stop-signal task. J Sport Health Sci. 2015;4:73–81. [Google Scholar]
- 20.Ramautar J.R., Kok A., Ridderinkhof K.R. Effects of stop-signal probability in the stop-signal paradigm: the N2/P3 complex further validated. Brain Cogn. 2004;56:234–252. doi: 10.1016/j.bandc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Bekker E.M., Kenemans J.L., Hoeksma M.R., Talsma D., Verbaten M.N. The pure electrophysiology of stopping. Int J Psychophysiol. 2005;55:191–198. doi: 10.1016/j.ijpsycho.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Swick D., Ashley V., Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- 23.Rubia K., Russell T., Overmeyer S., Brammer M.J., Bullmore E.T., Sharma T. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 24.Falkenstein M., Hoormann J., Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol (Amst) 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- 25.Roche R.A., Garavan H., Foxe J.J., O'Mara S.M. Individual differences discriminate event-related potentials but not performance during response inhibition. Exp Brain Res. 2005;160:60–70. doi: 10.1007/s00221-004-1985-z. [DOI] [PubMed] [Google Scholar]
- 26.Ramautar J.R., Kok A., Ridderinkhof K.R. Effects of stop-signal modality on the N2/P3 complex elicited in the stop-signal paradigm. Biol Psychol. 2006;72:96–109. doi: 10.1016/j.biopsycho.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Hillman C.H., Pontifex M.B., Motl R.W., O'Leary K.C., Johnson C.R., Scudder M.R. From ERPs to academics. Dev Cogn Neurosci. 2012;2(Suppl. 1):S90–S98. doi: 10.1016/j.dcn.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kok A., Ramautar J.R., De Ruiter M.B., Band G.P., Ridderinkhof K.R. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology. 2004;41:9–20. doi: 10.1046/j.1469-8986.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- 29.Senderecka M., Grabowska A., Szewczyk J., Gerc K., Chmylak R. Response inhibition of children with ADHD in the stop-signal task: an event-related potential study. Int J Psychophysiol. 2012;85:93–105. doi: 10.1016/j.ijpsycho.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Faul F., Erdfelder E., Buchner A., Lang A.G. Statistical power analyses using G * Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 31.Kaernbach C. Simple adaptive testing with the weighted up-down method. Percept Psychophys. 1991;49:227–229. doi: 10.3758/bf03214307. [DOI] [PubMed] [Google Scholar]
- 32.Verbruggen F., Logan G.D. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci Biobehav Rev. 2009;33:647–661. doi: 10.1016/j.neubiorev.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Band G.P., van der Molen M.W., Logan G.D. Horse-race model simulations of the stop-signal procedure. Acta Psychol (Amst) 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- 34.Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Cong F., Ristaniemi T., Lyytinen H. Advanced signal processing on brain event-related potentials: filtering ERPs in time, frequency and space domains sequentially and simultaneously. World Scientific; Singapore: 2015. pp. 15–23. [Google Scholar]
- 36.Wang C.H., Chang C.C., Liang Y.M., Shih C.M., Chiu W.S., Tseng P. Open vs. closed skill sports and the modulation of inhibitory control. PLoS One. 2013;8:e55773. doi: 10.1371/journal.pone.0055773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boecker M., Drueke B., Vorhold V., Knops A., Philippen B., Gauggel S. When response inhibition is followed by response reengagement: an event-related fMRI study. Hum Brain Mapp. 2011;32:94–106. doi: 10.1002/hbm.21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nieuwenhuis S., Yeung N., van den Wildenberg W., Ridderinkhof K.R. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- 39.Huster R.J., Plis S.M., Lavallee C.F., Calhoun V.D., Herrmann C.S. Functional and effective connectivity of stopping. Neuroimage. 2014;94:120–128. doi: 10.1016/j.neuroimage.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 40.Jahfari S., Waldorp L., Ridderinkhof K.R., Scholte H.S. Visual information shapes the dynamics of corticobasal ganglia pathways during response selection and inhibition. J Cogn Neurosci. 2015;27:1344–1359. doi: 10.1162/jocn_a_00792. [DOI] [PubMed] [Google Scholar]
- 41.Vogel E.K., Luck S.J. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37:190–203. [PubMed] [Google Scholar]
- 42.Callan D.E., Naito E. Neural processes distinguishing elite from expert and novice athletes. Cogn Behav Neurol. 2014;27:183–188. doi: 10.1097/WNN.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 43.Kato K., Iwamoto K., Kawano N., Noda Y., Ozaki N., Noda A. Differential effects of physical activity and sleep duration on cognitive function in young adults. J Sport Health Sci. 2018;7:227–236. doi: 10.1016/j.jshs.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.