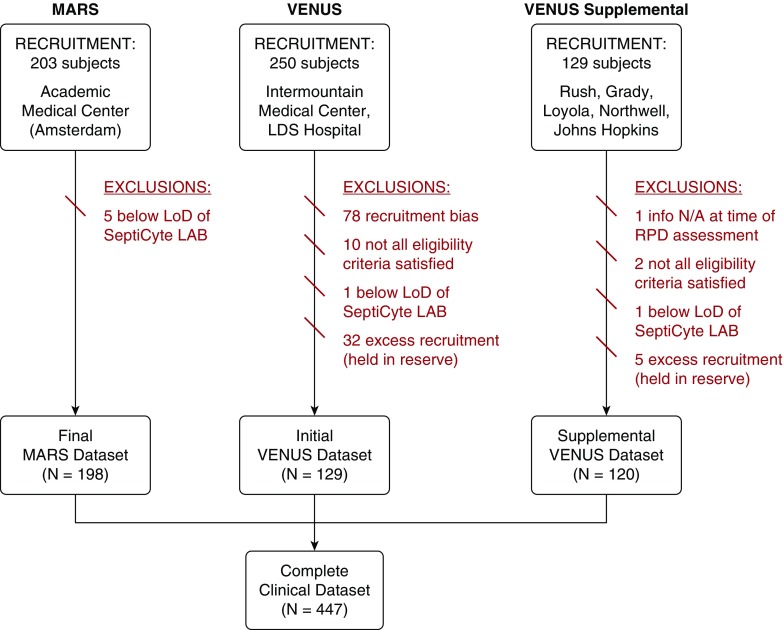
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram for the complete clinical dataset (N = 447) used in validation of SeptiCyte LAB. Subject recruitment dates were as follows: MARS (Molecular Diagnosis and Risk Stratification of Sepsis), between June 2013 and November 2013; VENUS (Validation of Septic Gene Expression Using SeptiCyte), between May 2014 and April 2015; VENUS Supplement, between March 2016 and August 2016. LoD = limit of detection; N/A = not applicable; RPD = retrospective physician diagnosis.