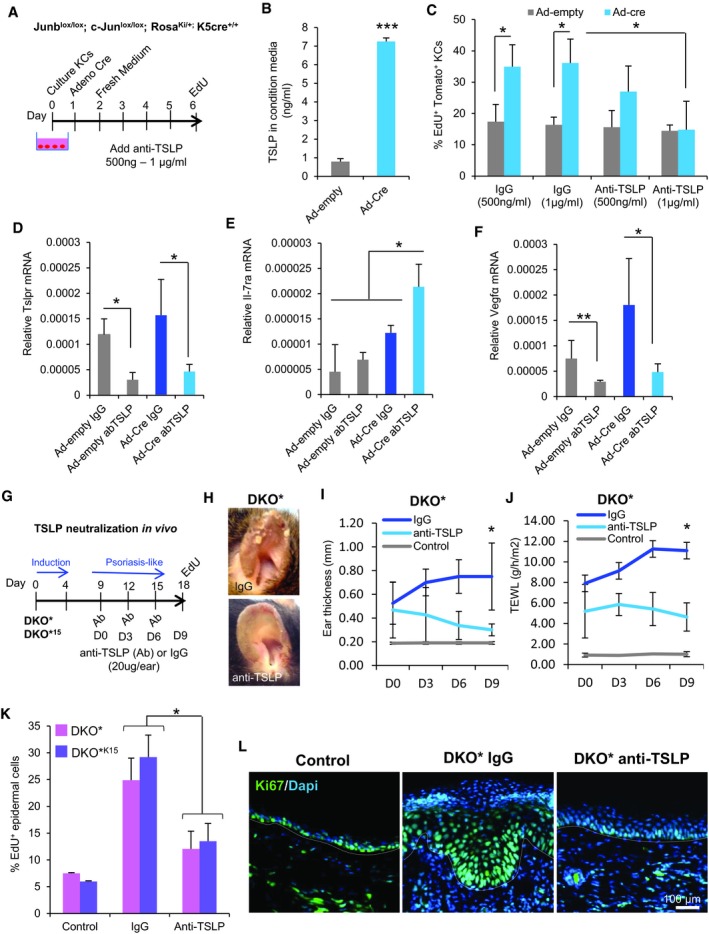
Figure 6. TSLP secreted by mutantGFP epidermal cells induces hyper‐proliferation of non‐mutantTom keratinocytes.
-
AExperimental design for induction of mutantGFP KCs in vitro and neutralization of secreted TSLP by anti‐TSLP. Primary keratinocytes were cultured from the skin of Junblox/lox; c‐Junlox/lox; RosaKi/+; and K5cre‐ERT+/+ mice. 50% of mutantGFP KCs were induced by infection with Cre Adenovirus, the non‐infected cells were non‐mutantTom KCs. Adeno‐empty was used as control. EdU was added for 5 h to label proliferating Tomato+ cells in 6‐day co‐culture. Quantification was performed by confocal imaging analysis.
-
BTSLP production in conditioned media quantified by ELISA three days after infection with Ad‐empty or Ad‐cre. n = 3 independent experiments. Data represent mean ± SD. Statistical significance ***P < 0.001 (Student's two‐tailed t‐test relative to control group). See Appendix Table S2 for exact P‐values.
-
CPercentage of EdU+ Tomato+ keratinocytes after blocking with anti‐TSLP. Anti‐TSLP neutralizes the hyper‐proliferation of non‐mutantTom KCs in vitro after infection with Ad‐cre. n = 3 independent experiments, six fields per experiment. Data represent mean ± SD. Statistical significance *P < 0.05 (two‐way ANOVA and Bonferroni post‐test).
-
DGene expression of Tslpr after TSLP neutralization is reduced. n = 3 independent experiments. Data represent mean ± SD. Statistical significance *P < 0.05 (Student's two‐tailed t‐test relative to control group). See Appendix Table S2 for exact P‐value.
-
EGene expression of IL‐7ra after TSLP neutralization is increased. n = 3 independent experiments. Data represent mean ± SD. Statistical significance *P < 0.05 (Student's two‐tailed t‐test relative to control group). See Appendix Table S2 for exact P‐value.
-
FGene expression of VEGFα after TSLP neutralization is reduced. n = 3 independent experiments. Data represent mean ± SD. Statistical significance *P < 0.05, **P < 0.01 (Student's two‐tailed t‐test relative to control groups). See Appendix Table S2 for exact P‐value.
-
GExperimental design for TSLP neutralization in vivo. TSLP neutralization was performed by triple intradermal injections of 20 μg of anti‐TSLP or IgG to the right ears of DKO* and DKO*15 mice 5 days after psoriasis‐like induction. EdU was added into mice by IP injection 2 h before euthanasia.
-
HRepresentative images of treated ears after 9 days of anti‐TSLP or IgG treatment.
-
I, JLongitudinal analysis of ear thickness (I) and trans‐epidermal water loss (TEWL) (J) in DKO* mice at different time points during psoriasis‐like disease progression. Data represent mean ± SD. n = 4. Statistical significance *P < 0.05 (two‐way ANOVA and Bonferroni post‐test).
-
KPercentage of EdU+ epidermal cells (CD45−CD49f+) after blocking with anti‐TSLP in DKO* and DKO*15 mice. DKO* n = 4 and DKO*15 n = 3 per group. Data represent mean ± SD. Statistical significance *P < 0.05 (two‐way ANOVA and Bonferroni post‐test).
-
LRepresentative immunofluorescence images of ear sections from control or DKO* mice treated with IgG or anti‐TSLP and stained for Ki67. Ki67 expression was reduced in mice treated with anti‐TSLP (n = 3).
Source data are available online for this figure.
