Abstract
Despite the beneficial effects of omega-3 fatty acids from fish or fish oil on cardiovascular diseases, limited information is available regarding the effects of oily fish in the diet on the risk of dyslipidemia. This study aimed to investigate the association between oily fish consumption and the incidence of dyslipidemia among Korean adults included in the Health Examinees Gem (HEXA-G) cohort during 5 years of follow-up. In total, 20,670 participants (5710 men and 14,960 women) were included in this study. The average intake of oily fish including dark meat fish, such as mackerel, pacific saury, and Spanish mackerel, and eel, was estimated using food frequency questionnaires. Oily fish consumption was associated with a significantly lower risk of hypertriglyceridemia in both men (Relative risk (RR) comparing extreme quintiles = 0.75; 95% CI 0.60–0.95; P for trend = 0.0121) and women (RR comparing extreme quintiles = 0.81; 95% CI 0.69–0.96; P for trend = 0.0110) after adjusting for potential confounders. In conclusion, increased consumption of oily fish was significantly associated with a lower risk of hypertriglyceridemia in the general Korean population. Future randomized clinical trials or prospective studies are required to confirm these findings in the Korean or other Asian populations.
Keywords: fish, oily fish, omega-3 fatty acid, dyslipidemia, hypertriglyceridemia
1. Introduction
Dyslipidemia is a metabolic anomaly distinguished by an increase or reduction in the plasma lipid fraction [1]. Generally, high levels of low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), triglycerides (TG), and low levels of high-density lipoprotein cholesterol (HDL-C) are the primary risk factors of atherosclerosis, leading to cardiovascular disease (CVD) and coronary heart disease (CHD) [2]. The prevalence of dyslipidemia has continuously increased not only in Korea but also worldwide. An estimated 53% and 44% of the U.S. and Korean adults, respectively, have dyslipidemia [3,4]. Among the blood lipid components, LDL-C is the most important risk factor in the pathophysiology of CVD, and reducing LDL-C levels leads to dose-dependent reduction in the risk of major cardiovascular events that is proportional to the absolute magnitude of the reduction in LDL-C [5]. Furthermore, a meta-analysis of controlled trials reported that a 1 mmol/L reduction in TG was associated with reduction in coronary events by 54% for the overall population and by 43% in those with high TG levels [6]. Thus, improvement in the lipid profile is important to prevent CVD.
Nutritional and lifestyle modifications form the basis for treating dyslipidemia to prevent and reduce the risk of CVD [7]. Many epidemiological studies have reported the beneficial effects of omega-3 poly-unsaturated fatty acids (PUFAs) on cardiovascular health [8,9] The cardioprotective effects of omega-3 PUFAs, particularly in terms of blood lipid, can be explained by mechanisms such as increased clearance and decreased hepatic very-low-density lipoprotein production rates [8]. Fish is the primary dietary source of omega-3 PUFAs. Among omega-3 PUFAs, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) have been most widely studied for their beneficial effects on cardiovascular health [8,9]. Furthermore, these fatty acids reduce blood pressure, serum TG, and glucose levels, and increase HDL-C levels [10,11]. Other than PUFAs, such as DHA and EPA, fish also includes vitamin D and high-quality proteins, which may have beneficial effects against dyslipidemia [12,13].
Despite numerous epidemiological studies on the beneficial effects of omega-3 fatty acids from fish or fish oil against CVD, limited information is available regarding the effects of oily fish in the diet on the risk of dyslipidemia. Oily fish, such as mackerel, tuna, salmon, sardines, and herring, are the primary dietary sources of omega-3 fatty acids, particularly DHA and EPA [8,9]. Therefore, oily fish may have more potent effects on the risk of dyslipidemia when compared to non-oily fish.
Moreover, although fish is a major part of the Korean diet, accounting for approximately 20% of the energy intake from animal sources owing to its peninsular characteristics [14], limited information is available regarding the effects of fish consumption against the risk of dyslipidemia. The purpose of the present study was to examine the association between oily fish consumption and the incidence of dyslipidemia among Korean adults included in the Health Examinees Gem (HEXA-G) Study cohort during 5 years of follow-up.
2. Materials and Methods
2.1. Study Population
The HEXA study [15,16] is a large-scale community-based prospective cohort study conducted in Korea. The baseline survey of HEXA study was conducted from 2004 to 2013. In total, 169,722 participants aged 40–69 years were recruited in 38 general hospitals and health examination centers in eight regions in Korea. Based on previous HEXA studies, this study used data of the individuals included in the HEXA-G cohort, for which additional eligibility criteria were applied (i.e., hospitals or health examination centers). Of the original 38 sites, 30,374 individuals at 21 sites were excluded in accordance with previously reported exclusion criteria [17]. Thus far, 64,486 participants of the HEXA-G study completed the initial follow-up survey between 2012 and 2016. Among them, those with dyslipidemia (n = 40,983), diabetes (n = 1,277), or cardiovascular diseases (n = 597) at baseline and with insufficient data regarding blood lipid profiles at baseline (n = 3) and at follow-up (n = 6), implausible energy intake (< 800 or ≥ 4000 kcal/day in men and < 500 or ≥ 3500 kcal/day in women) (n = 938), and those with insufficient information regarding body mass index (BMI) (n = 12) were excluded. Finally, 20,670 participants (5710 men and 14,960 women) were included in this study. The HEXA-G study was approved by the Ethics Committee of the Korean Health and the institutional review boards of all participating hospitals (IRB No. E-1503-103-657). All participants provided informed written consent prior to participating the study.
2.2. Diet Assessment
The validated semi-quantitative food frequency questionnaire (FFQ), including 106 food items, was used at baseline and follow-up examinations to assess oily fish consumption and nutrient intake among participants. The participants were asked how often they consumed each food item on average during the past year. Daily nutrient intake was calculated by multiplying the frequency of consumption of each food item by its nutrient content and summing up the nutrient intake from all food items. Of the 14 fish items, oily fish comprised dark-meat fish, including mackerel, pacific saury, and Spanish mackerel, and eel. The cumulative average intake of oily fish was estimated using the first (at baseline) and second (at follow-up) FFQs to represent long-term intake.
2.3. Definition of Dyslipidemia
The end point for this study was the occurrence of dyslipidemia and its components, including hypercholesterolemia, hyper-LDL cholesterolemia, hypo-HDL cholesterolemia, and hypertriglyceridemia occurring after baseline but before follow-up examination. Dyslipidemia and its components were diagnosed on the basis of the analysis of blood samples obtained after 8 h of fasting.
Participants satisfying one of the following four components were diagnosed as having dyslipidemia. Hypercholesterolemia was defined as a blood TC level ≥ 200 mg/dL; hyper-LDL cholesterolemia as blood LDL-C ≥ 130 mg/dL; hypo-HDL cholesterolemia as HDL-C level < 40 mg/dL; and hypertriglyceridemia as TG level ≥ 150 mg/dL.
2.4. Assessment of Other Variables
Body mass index (BMI) was determined by dividing the body weight by the square of the height (kg/m2). BMI values were used to classify participants into the following groups: underweight (BMI < 18.5 kg/m2), normal (BMI ≥ 18.5 kg/m2 and < 23 kg/m2), overweight (BMI ≥ 23 kg/m2 and < 25 kg/m2), and obese (BMI ≥ 25 kg/m2) in accordance with the International Obesity Task Force for adults in Asian and Pacific regions [18]. Sociodemographic variables, such as age, sex, and education level, and lifestyle variables, such as alcohol consumption, current smoking status, and physical activity, were obtained through self-administered questionnaires. Education level was divided into three categories: under middle school, high school, and over college. Alcohol consumption was divided into two categories: “current drinker” (drank alcohol at the time of survey) or “non-drinker” (never drank alcohol or have abstained from alcohol). Current smoking status was divided into three categories: “current smoker” (smoked cigarettes at the time of survey), “past smoker” (have abstained from cigarettes smoking), or “never smoker” (never smoked cigarettes). Physical activity was divided into two categories: “active” (performed physical activity for ≥ 30 min once a day for ≥ 5 d a week) or “inactive.”
2.5. Statistical Analysis
For each participant, person-years of follow-up were calculated from baseline to the first follow-up examination. We calculated the incidence of dyslipidemia and its components per person years. We performed Cox proportional hazards regression analysis to estimate the relative risks (RRs) and 95% confidence interval (CI) of dyslipidemia in accordance with the quintiles of oily fish consumption after adjusting for potential confounders, such as age (continuous), BMI (continuous), education level, alcohol consumption, current smoking status, physical activity, and energy intake (continuous) by sex. Subgroup analysis was performed for dyslipidemia and its components, which revealed significant associations with oily fish consumption. We estimated the RR and 95% CI of the fifth quintile (Q5) in comparison with the first quintile (Q1) by sex in accordance with baseline age, baseline BMI level, and menopausal status (in women). All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA) and a two-sided p-value < 0.05 was considered statistically significant.
3. Results
During the 5 years of follow-up (total 104,927 person-years), we documented 8751 incident cases of dyslipidemia (7127 cases of hypercholesterolemia, 3923 cases of hyper-LDL cholesterolemia, 674 cases of hypo-HDL cholesterolemia, and 2287 cases of hypertriglyceridemia).
The general characteristics of the study population at baseline in accordance with the quintiles of oily fish consumption by sex are shown in Table 1. In both men and women, participants with higher oily fish consumption tended to have a higher education level and higher BMI level, consume alcohol, performe physical activity, and consume more energy and macronutrients, including carbohydrate, protein, and fat, in comparison with those with a lower consumption (all P < 0.001).
Table 1.
General characteristics of the study population at baseline examination in accordance with the quintiles of oily fish consumption by sex in the HEXA-G study.
| Men | Women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p Value | Q1 | Q2 | Q3 | Q4 | Q5 | p value | |
| N | 1120 | 1146 | 1239 | 1089 | 1116 | 3044 | 2940 | 2989 | 2983 | 3004 | ||
| Age (years) | 55.2 ± 8.6 | 54.2 ± 8.2 | 54.7 ± 8.2 | 53.9 ± 8.5 | 53.8 ± 8.1 | <0.0001 | 50.2 ± 7.5 | 49.7 ± 7.2 | 50.0 ± 7.4 | 49.4 ± 7.1 | 50.3 ± 7.0 | <0.0001 |
| Age (years), n (%) | ||||||||||||
| 40–49 | 312 (27.9) | 348 (30.4) | 351 (28.3) | 361 (33.2) | 365 (32.7) | <0.0010 | 1564 (51.4) | 1561 (53.1) | 1536 (51.4) | 1654 (55.5) | 1491 (49.6) | <0.0001 |
| 50–59 | 389 (34.7) | 435 (38.0) | 477 (38.5) | 405 (37.2) | 423 (37.9) | 1044 (34.3) | 1045 (35.5) | 1065 (35.6) | 1005 (33.7) | 1145 (38.1) | ||
| 60–69 | 419 (37.4) | 363 (31.7) | 411 (33.2) | 323 (29.7) | 328 (29.4) | 436 (14.3) | 334 (11.4) | 388 (13.0) | 324 (10.9) | 368 (12.3) | ||
| Education level, n (%) | ||||||||||||
| Under middle school | 321 (28.7) | 241 (21.0) | 272 (22.0) | 213 (19.6) | 184 (16.5) | <0.0001 | 1032 (33.9) | 811 (27.6) | 874 (29.2) | 738 (24.7) | 751 (25.0) | <0.0001 |
| High school | 413 (36.9) | 473 (41.3) | 485 (39.1) | 431 (39.6) | 472 (42.3) | 1314 (43.2) | 1382 (47.0) | 1374 (46.0) | 1493 (50.1) | 1497 (49.8) | ||
| Over college | 370 (33.0) | 419 (36.6) | 466 (37.6) | 435 (39.9) | 448 (40.1) | 665 (21.9) | 716 (24.4) | 706 (23.6) | 729 (24.4) | 719 (23.9) | ||
| BMI (kg/m2) | 23.2 ± 2.7 | 23.4 ± 2.6 | 23.5 ± 2.6 | 23.8 ± 2.6 | 23.9 ± 2.6 | <0.0001 | 22.7 ± 2.7 | 22.9 ± 2.8 | 22.9 ± 2.7 | 23.0 ± 2.7 | 23.1 ± 2.7 | <0.0001 |
| Obesity, n (%) | ||||||||||||
| Underweight | 37 (3.3) | 29 (2.5) | 26 (2.1) | 15 (1.4) | 20 (1.8) | <0.0001 | 126 (4.1) | 94 (3.2) | 81 (2.7) | 78 (2.6) | 66 (2.2) | 0.0003 |
| Normal | 497 (44.4) | 476 (41.5) | 500 (40.4) | 425 (39.0) | 407 (36.5) | 1623 (53.3) | 1590 (54.1) | 1577 (52.8) | 1571 (52.7) | 1525 (50.8) | ||
| Overweight | 314 (28.0) | 350 (30.5) | 393 (31.7) | 301 (27.6) | 301 (27.0) | 706 (23.2) | 680 (23.1) | 743 (24.9) | 749 (25.1) | 779 (25.9) | ||
| Obese | 272 (24.3) | 291 (25.4) | 320 (25.8) | 348 (32.0) | 388 (34.8) | 589 (19.4) | 576 (19.6) | 588 (19.7) | 585 (19.6) | 634 (21.1) | ||
| Alcohol consumption | ||||||||||||
| Non-drinker | 377 (33.7) | 298 (26.0) | 332 (26.8) | 252 (23.1) | 254 (22.8) | <0.0001 | 2104 (69.1) | 1925 (65.5) | 1966 (65.8) | 1893 (63.5) | 2008 (66.8) | 0.0008 |
| Current drinker | 740 (66.1) | 847 (73.9) | 901 (72.7) | 831 (76.3) | 857 (76.8) | 929 (30.5) | 1001 (34.1) | 1011 (33.8) | 1081 (36.2) | 979 (32.6) | ||
| Current smoking status | ||||||||||||
| Never smoker | 410 (36.6) | 423 (36.9) | 436 (35.2) | 364 (33.4) | 366 (32.8) | 0.5620 | 2955 (97.1) | 2858 (97.2) | 2914 (97.5) | 2910 (97.6) | 2908 (96.8) | 0.5502 |
| Past smoker | 448 (40.0) | 458 (40.0) | 508 (41.0) | 445 (40.9) | 455 (40.8) | 28 (0.9) | 29 (1.0) | 23 (0.8) | 24 (0.8) | 37 (1.2) | ||
| Current smoker | 258 (23.0) | 261 (22.8) | 289 (23.3) | 276 (25.3) | 287 (25.7) | 51 (1.7) | 40 (1.4) | 40 (1.3) | 39 (1.3) | 40 (1.3) | ||
| Physical activity | ||||||||||||
| Active | 217 (19.4) | 224 (19.6) | 241 (19.5) | 237 (21.8) | 288 (25.8) | 0.0002 | 485 (15.9) | 467 (15.9) | 522 (17.5) | 520 (17.4) | 651 (21.7) | <0.0001 |
| Inactive | 885 (79.0) | 895 (78.1) | 960 (77.5) | 819 (75.2) | 793 (71.1) | 2519 (82.8) | 2426 (82.5) | 2425 (81.1) | 2390 (80.1) | 2274 (75.7) | ||
| Nutrient intake | ||||||||||||
| Total energy (Kcal/d) | 1658.6 ± 436.3 | 1754.3 ± 437.2 | 1816.8 ± 406.4 | 1913.0 ± 460.6 | 2057.5 ± 492.8 | <0.0001 | 1534.4 ± 431.2 | 1600.8 ± 445.5 | 1678.4 ± 456.7 | 1758.0 ± 481.0 | 1921.0 ± 7.0 | <0.0001 |
| Carbohydrates (g/d) | 309.0 ± 76.8 | 317.6 ± 77.6 | 326.5 ± 72.4 | 335.4 ± 78.4 | 348.8 ± 80.1 | <0.0001 | 286.4 ± 79.8 | 292.9 ± 82.3 | 301.4 ± 82.1 | 310.2 ± 85.5 | 327.3 ± 90.6 | <0.0001 |
| Protein (g/d) | 49.3 ± 16.8 | 56.0 ± 17.6 | 59.5 ± 16.9 | 65.6 ± 20.5 | 76.7 ± 26.0 | <0.0001 | 46.7 ± 16.0 | 51.1 ± 16.6 | 56.0 ± 18.5 | 60.8 ± 19.4 | 72.2 ± 25.3 | <0.0001 |
| Fat (g/d) | 22.3 ± 13.1 | 26.3 ± 13.0 | 27.8 ± 12.4 | 32.0 ± 14.8 | 37.4 ± 17.1 | <0.0001 | 20.6 ± 11.6 | 23.2 ± 12.1 | 26.0 ± 13.3 | 29.0 ± 140 | 34.8 ± 17.0 | < .0001 |
HEXA-G: Health Examinees Gem.
Table 2 shows the RRs of dyslipidemia in accordance with the quintiles of oily fish consumption by sex. The RR for hypertriglyceridemia among individuals with the highest oily fish consumption was 0.75 (95% CI 0.60–0.95, P for trend = 0.0121) in men and 0.81 (95% CI 0.69–0.96, P for trend = 0.0110) in women after adjusting for age, BMI, education level, alcohol consumption, current smoking status, physical activity, and energy intake in comparison with those for individuals with the lowest consumption. However, there was no significant association between oily fish consumption and other components of dyslipidemia.
Table 2.
Relative risks of dyslipidemia in accordance with the quintiles of oily fish consumption by sex in the HEXA-G study.
| Oily Fish Consumption (g/d) | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P for Trend | |
| Men (n = 5710) | ||||||
| Median (range) | 1.7 (0.0–2.6) | 3.5 (2.7–4.9) | 6.0 (5.0-6.8) | 8.6 (6.8-10.7) | 16.0 (10.7-116.4) | |
| Person-years, mean (sum) | 5.0 (5579.4) | 4.9 (5658.3) | 5.0 (6138.7) | 4.9 (5350.0) | 5.0 (5589.6) | |
| Hypercholesterolemia | ||||||
| Cases, n | 287 | 292 | 313 | 275 | 313 | |
| Model 1 | 1.00 (Ref.) | 1.00 (0.85-1.18) | 0.97 (0.83-1.14) | 1.00 (0.85-1.19) | 1.06 (0.90-1.24) | 0.3829 |
| Model 2 | 1.00 (Ref.) | 0.99 (0.84-1.17) | 0.96 (0.82-1.13) | 0.99 (0.84-1.17) | 1.04 (0.88-1.23) | 0.5078 |
| Hyper-LDL cholesterolemia | ||||||
| Cases, n | 164 | 155 | 168 | 174 | 186 | |
| Model 1 | 1.00 (Ref.) | 0.92 (0.74-1.15) | 0.90 (0.73-1.12) | 1.11 (0.89-1.37) | 1.09 (0.88-1.35) | 0.1160 |
| Model 2 | 1.00 (Ref.) | 0.92 (0.74-1.15) | 0.90 (0.72-1.12) | 1.11 (0.89-1.38) | 1.10 (0.88-1.37) | 0.1072 |
| Hypo-HDL cholesterolemia | ||||||
| Cases, n | 65 | 51 | 80 | 55 | 47 | |
| Model 1 | 1.00 (Ref.) | 0.75 (0.52-1.09) | 1.06 (0.76-1.47) | 0.84 (0.59-1.21) | 0.66 (0.45-0.96) | 0.0507 |
| Model 2 | 1.00 (Ref.) | 0.79 (0.55-1.14) | 1.11 (0.79-1.54) | 0.87 (0.60-1.27) | 0.68 (0.46-1.01) | 0.0762 |
| Hypertriglyceridemia | ||||||
| Cases, n | 159 | 166 | 175 | 163 | 146 | |
| Model 1 | 1.00 (Ref.) | 0.99 (0.80-1.23) | 0.93 (0.75-1.16) | 0.99 (0.79-1.23) | 0.80 (0.64-1.01) | 0.0419 |
| Model 2 | 1.00 (Ref.) | 0.98 (0.78-1.21) | 0.91 (0.73-1.13) | 0.94 (0.75-1.17) | 0.75 (0.60-0.95) | 0.0121 |
| Dyslipidemia | ||||||
| Cases, n | 418 | 420 | 462 | 411 | 431 | |
| Model 1 | 1.00 (Ref.) | 0.98 (0.86-1.12) | 0.98 (0.85-1.11) | 1.01 (0.89-1.16) | 0.98 (0.86-1.13) | 0.9710 |
| Model 2 | 1.00 (Ref.) | 0.97 (0.85-1.12) | 0.97 (0.84-1.11) | 1.00 (0.87-1.14) | 0.96 (0.84-1.11) | 0.7353 |
| Women (n = 14,960) | ||||||
| Median (range) | 1.5 (0.0-2.3) | 3.3 (2.3-4.3) | 5.8 (4.3-6.7) | 8.5 (6.8-10.7) | 15.0 (10.7-102.1) | |
| Person-years, mean (sum) | 5.0 (15232.7) | 5.1 (14975.6) | 5.1 (15171.8) | 5.1 (15261.0) | 5.3 (15969.5) | |
| Hypercholesterolemia | ||||||
| Cases, n | 1104 | 1080 | 1081 | 1156 | 1226 | |
| Model 1 | 1.00 (Ref.) | 0.97 (0.89-1.06) | 0.95 (0.87-1.03) | 1.02 (0.94-1.11) | 0.97 (0.89-1.05) | 0.7576 |
| Model 2 | 1.00 (Ref.) | 0.96 (0.89-1.05) | 0.94 (0.86-1.02) | 1.00 (0.92-1.09) | 0.97 (0.89-1.05) | 0.8116 |
| Hyper-LDL cholesterolemia | ||||||
| Cases, n | 603 | 598 | 575 | 617 | 683 | |
| Model 1 | 1.00 (Ref.) | 0.98 (0.88-1.10) | 0.91 (0.81-1.02) | 0.99 (0.88-1.11) | 0.97 (1.01-1.02) | 0.7691 |
| Model 2 | 1.00 (Ref.) | 0.97 (0.87-1.09) | 0.91 (0.81-1.02) | 0.98 (0.88-1.10) | 0.97 (0.87-1.09) | 0.9111 |
| Hypo-HDL cholesterolemia | ||||||
| Cases, n | 76 | 81 | 70 | 72 | 77 | |
| Model 1 | 1.00 (Ref.) | 1.05 (0.77-1.44) | 0.87 (0.63-1.20) | 0.91 (0.66-1.26) | 0.84 (0.61-1.16) | 0.1915 |
| Model 2 | 1.00 (Ref.) | 1.05 (0.77-1.44) | 0.87 (0.63-1.20) | 0.91 (0.66-1.26) | 0.83 (0.60-1.15) | 0.1699 |
| Hypertriglyceridemia | ||||||
| Cases, n | 320 | 297 | 294 | 267 | 300 | |
| Model 1 | 1.00 (Ref.) | 0.92 (0.78-1.08) | 0.87 (0.74-1.02) | 0.80 (0.68-0.95) | 0.79 (0.67-0.92) | 0.0024 |
| Model 2 | 1.00 (Ref.) | 0.92 (0.78-1.08) | 0.88 (0.75-1.04) | 0.81 (0.69-0.96) | 0.81 (0.69-0.96) | 0.0110 |
| Dyslipidemia | ||||||
| Cases, n | 1299 | 1294 | 1263 | 1329 | 1424 | |
| Model 1 | 1.00 (Ref.) | 0.99 (0.92-1.07) | 0.94 (0.87-1.01) | 0.99 (0.92-1.07) | 0.95 (0.88-1.02) | 0.2516 |
| Model 2 | 1.00 (Ref.) | 0.98 (0.91-1.06) | 0.93 (0.86-1.01) | 0.98 (0.91-1.06) | 0.95 (0.88-1.03) | 0.3061 |
HEXA-G: Health Examinees Gem; RR (95% CI); Model 1: Adjusted for age (continuous), and BMI (continuous); Model 2: Additionally adjusted for education level (under middle school, high school, or over college), smoking status (never, past, or current smoker), alcohol consumption (non-drinker or current drinker), physical activity (yes or no), energy intake (continuous).
Table 3 shows the RRs of dyslipidemia in accordance with the quintiles of total fish consumption by sex. Although a significant positive trend was observed between total fish consumption and the risk of hyper-LDL cholesterolemia in men after adjusting for potential confounders (P for trend = 0.0074), no association was observed between total fish consumption and the risk of dyslipidemia and its components.
Table 3.
Relative risks of dyslipidemia in accordance with the quintiles of total fish consumption by sex in the HEXA-G study.
| Total Fish Consumption (g/d) | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P for Trend | |
| Men (n = 5710) | ||||||
| Median (range) | 11.9 (0.2-16.8) | 21.0 (16.8–25.1) | 29.6 (25.1–34.8) | 40.8 (34.8–49.9) | 65.5 (49.9–292.2) | |
| Person-years, mean (sum) | 5.0 (5468.7) | 4.9 (5472.3) | 4.9 (5553.5) | 5.0 (5870.7) | 4.9 (5950.8) | |
| Hypercholesterolemia | ||||||
| Cases, n | 261 | 279 | 308 | 319 | 313 | |
| Model 1 | 1.00 (Ref.) | 1.05 (0.88–1.24) | 1.14 (0.97–1.35) | 1.13 (0.96–1.33) | 1.12 (0.95–1.32) | 0.1948 |
| Model 2 | 1.00 (Ref.) | 1.04 (0.88–1.23) | 1.13 (0.96–1.34) | 1.12 (0.95–1.32) | 1.11 (0.93–1.33) | 0.2593 |
| Hyper-LDL cholesterolemia | ||||||
| Cases, n | 151 | 140 | 169 | 192 | 195 | |
| Model 1 | 1.00 (Ref.) | 0.91 (0.72–1.15) | 1.08 (0.87–1.35) | 1.17 (0.95–1.45) | 1.20 (0.97–1.49) | 0.0145 |
| Model 2 | 1.00 (Ref.) | 0.91 (0.72–1.15) | 1.10 (0.88–1.37) | 1.19 (0.96–1.49) | 1.25 (1.00–1.58) | 0.0074 |
| Hypo-HDL cholesterolemia | ||||||
| Cases, n | 68 | 53 | 48 | 74 | 55 | |
| Model 1 | 1.00 (Ref.) | 0.77 (0.53–1.10) | 0.68 (0.47–0.99) | 0.99 (0.71–1.37) | 0.72 (0.50–1.03) | 0.3026 |
| Model 2 | 1.00 (Ref.) | 0.80 (0.56–1.15) | 0.74 (0.51–1.08) | 1.03 (0.73–1.44) | 0.72 (0.49–1.06) | 0.3069 |
| Hypertriglyceridemia | ||||||
| Cases, n | 157 | 148 | 147 | 188 | 169 | |
| Model 1 | 1.00 (Ref.) | 0.90 (0.71–1.12) | 0.89 (0.71–1.11) | 1.06 (0.86–1.31) | 0.93 (0.75–1.15) | 0.9930 |
| Model 2 | 1.00 (Ref.) | 0.89 (0.71–1.12) | 0.88 (0.70–1.11) | 1.02 (0.82–1.27) | 0.87 (0.69–1.10) | 0.5172 |
| Dyslipidemia | ||||||
| Cases, n | 396 | 403 | 409 | 478 | 456 | |
| Model 1 | 1.00 (Ref.) | 1.00 (0.87–1.15) | 1.00 (0.87–1.15) | 1.11 (0.97–1.27) | 1.06 (0.92–1.21) | 0.2005 |
| Model 2 | 1.00 (Ref.) | 0.99 (0.86-1.14) | 0.99 (0.86–1.14) | 1.09 (0.95–1.26) | 1.04 (0.90–1.20) | 0.3559 |
| Women (n = 14,960) | ||||||
| Median (range) | 11.9 (0.0-16.8) | 21.0 (16.8–25.1) | 29.6 (25.1–34.8) | 41.0 (34.8–49.9) | 65.5 (49.9–348.5) | |
| Person-years, mean (sum) | 5.1 (15389.3) | 5.1 (15350.3) | 5.1 (15201.3) | 5.1 (15218.5) | 5.3 (15451.2) | |
| Hypercholesterolemia | ||||||
| Cases, n | 1099 | 1082 | 1152 | 1158 | 1156 | |
| Model 1 | 1.00 (Ref.) | 0.98 (0.90–1.06) | 1.08 (0.99–1.17) | 1.04 (0.96–1.13) | 0.99 (0.91–1.07) | 0.9380 |
| Model 2 | 1.00 (Ref.) | 0.97 (0.90–1.06) | 1.07 (0.99–1.17) | 1.04 (0.95-1.13) | 1.00 (0.91–1.09) | 0.9228 |
| Hyper-LDL cholesterolemia | ||||||
| Cases, n | 586 | 582 | 631 | 631 | 646 | |
| Model 1 | 1.00 (Ref.) | 0.98 (0.88–1.10) | 1.11 (0.99–1.24) | 1.06 (0.95–1.19) | 1.02 (0.91–1.14) | 0.6460 |
| Model 2 | 1.00 (Ref.) | 0.99 (0.88–1.11) | 1.11 (0.99–1.25) | 1.07 (0.95–1.20) | 1.05 (0.93–1.18) | 0.4159 |
| Hypo-HDL cholesterolemia | ||||||
| Cases, n | 87 | 71 | 64 | 73 | 81 | |
| Model 1 | 1.00 (Ref.) | 0.82 (0.60–1.12) | 0.76 (0.55–1.05) | 0.83 (0.60–1.13) | 0.84 (0.62–1.14) | 0.5000 |
| Model 2 | 1.00 (Ref.) | 0.81 (0.59–1.10) | 0.76 (0.55–1.05) | 0.81 (0.59–1.12) | 0.82 (0.59–1.14) | 0.4593 |
| Hypertriglyceridemia | ||||||
| Cases, n | 307 | 304 | 297 | 273 | 297 | |
| Model 1 | 1.00 (Ref.) | 0.99 (0.84–1.16) | 1.00 (0.85–1.17) | 0.88 (0.75–1.03) | 0.88 (0.75–1.04) | 0.0537 |
| Model 2 | 1.00 (Ref.) | 1.00 (0.85–1.17) | 1.02 (0.87–1.20) | 0.90 (0.76–1.07) | 0.93 (0.78–1.10) | 0.2375 |
| Dyslipidemia | ||||||
| Cases, n | 1299 | 1282 | 1344 | 1338 | 1346 | |
| Model 1 | 1.00 (Ref.) | 0.98 (0.91–1.06) | 1.06 (0.98–1.15) | 1.02 (0.94–1.10) | 0.97 (0.90–1.05) | 0.4501 |
| Model 2 | 1.00 (Ref.) | 0.98 (0.91–1.06) | 1.06 (0.98–1.15) | 1.02 (0.94–1.10) | 0.98 (0.90–1.06) | 0.6362 |
HEXA-G: Health Examinees Gem; RR (95% CI); Model 1: Adjusted for age (continuous), and BMI (continuous); Model 2: Additionally adjusted for education level (under middle school, high school, or over college), smoking status (never, past, or current smoker), alcohol consumption (non-drinker or current drinker), physical activity (yes or no), energy intake (continuous).
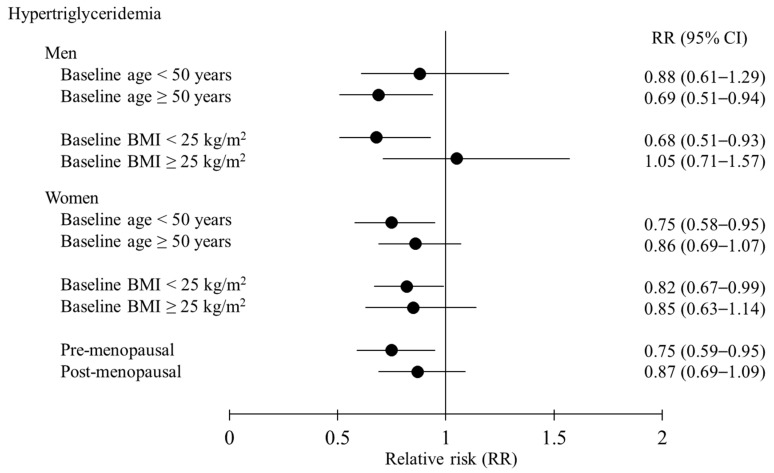
Figure 1 shows the RRs of hypertriglyceridemia on comparing extreme quintiles of oily fish consumption by sex in accordance with baseline age, baseline BMI level, and menopausal status (in women). The group with the highest oily fish consumption with a baseline age ≥ 50 years among men displayed a 31% lower risk of hypertriglyceridemia in comparison with the group with the lowest oily fish consumption, whereas, among women, the group with the highest oily fish consumption with a baseline age < 50 years displayed a 25% lower risk of hypertriglyceridemia in comparison with the group with the lowest oily fish consumption. Among both men and women, the group with the highest oily fish consumption with a baseline BMI < 25 kg/m2 displayed a 32% and 18%, respectively, lower risk of hypertriglyceridemia in comparison with the lowest oily fish consumption group. Among pre-menopausal women, the RR for hypertriglyceridemia in the group with the highest oily fish consumption was 25% lower than that of the group with the lowest consumption.
Figure 1.
Relative risks of hypertriglyceridemia when comparing extreme quintiles of oily fish consumption by sex in accordance with baseline age, baseline BMI level, and menopausal status (in women) in the HEXA-G study.
4. Discussion
This prospective cohort study, conducted on a population sample from Korea, a country with high fish consumption, shows a significant inverse association between oily fish consumption and the incidence of hypertriglyceridemia after a 5-year follow-up.
Numerous randomized controlled trials (RCTs) have reported that either a fish in the diet or fish oil supplements improve blood TG levels in healthy individuals or those with hyperlipidemia [8,9] and those with type 2 diabetes [19,20]. The present results showed no association between total fish consumption and the risk of dyslipidemia; however, a significant inverse association was observed between oily fish consumption and the risk of hypertriglyceridemia. The TG-lowering effect of fish consumption depends on the long chain omega-3 PUFA content in fish [8]. Therefore, the intake of oily fish high in omega-3 fatty acids, rather than total fish, can reduce risk of hypertriglyceridemia. The potential mechanism underlying the hypotriglyceridemic effect of oily fish can be explained on the basis of the role of omega-3 fatty acids in the inhibition of TG and hepatic very-low-density lipoprotein synthesis [21,22]. Because high blood TG levels are independent risk factors for CVD, such as CHD, ischemic stroke, and myocardial infarction [6], oily fish consumption can be beneficial for cardiovascular health.
Subgroup analyses (Figure 1) revealed that the TG-lowering effects of oily fish consumption were observed only at a baseline BMI < 25 kg/m2 (non-obese) and in pre-menopausal women. Elevated TG level is the most prominent feature of dyslipidemia in obesity [23]. The overload of hepatic TG leads to delayed clearance of the TG-rich lipoproteins and the formation of small dense LDL aggregates, thus increasing TG levels and subsequently resulting in dyslipidemia [23]. Thus, impaired lipid metabolism in obesity may negatively affect the TG-lowering effects of oily fish, resulting in a null association in obese individuals. Furthermore, postmenopausal women tend to have significantly different lipid profiles, higher concentrations of TC, TG, and LDL-C, but lower HDL-C levels in comparison with premenopausal women [24]. Owing to these differences, these associations may be significant only among pre-menopausal women.
However, although omega-3 PUFAs usually decrease blood TG levels, their effects on TC, LDL-C, and HDL-C levels are subtle and conflicting [25]. A systematic review and meta-analysis that summarized 47 RCTs reported that fish oil supplementation significantly reduced blood TG levels but not TC, HDL-C, or LDL-C levels in hyperlipidemia patients [26]. Rather, LDL-C levels increased slightly but significantly after fish oil supplementation [26]. Similarly, in the present study, oily fish consumption did not significantly alter the risk of hypercholesterolemia, hyper-LDL cholesterolemia, and hypo-HDL cholesterolemia; however, a significant positive trend was observed between total fish consumption and the risk of hyper-LDL cholesterolemia.
Although the beneficial effects of omega-3 fatty acids from fish or fish oil against CVD risk factors, including dyslipidemia, have been widely reported, limited information is available regarding the effects of oily fish consumption on the risk of dyslipidemia. In an 8-week RCT in China, oily fish consumption, including salmon, significantly reduced serum TG levels and increased HDL-C levels in adult men with hypertriglyceridemia [27], while it did not alter blood lipid levels among Chinese adult women with hypertriglyceridemia in the subsequent 8-week trial [28]. However, in the present study, a higher consumption of oily fish, including dark-meat fish (mackerel, pacific saury, and Spanish mackerel) and eel, was associated with a lower risk of hypertriglyceridemia; however, it was not associated with the risk of hypercholesterolemia, hyper-LDL cholesterolemia, and hypo-HDL cholesterolemia. The differences in oily fish species, content of EPA + DHA in fish, and study population among these studies may have resulted in these inconsistencies.
This may lead to questions regarding the optimal source of omega-3 fatty acids for foods or supplements. The optimal dose of omega-3 PUFAs to reduce the risk of dyslipidemia is yet unclear. Fish, especially oily fish, contains not only sufficient omega-3 PUFAs but also various nutrients, including high-quality proteins, vitamin D, selenium, and other minerals and elements [9]. Some nutrients in fish, such as vitamin E and selenium, also have beneficial roles to offset the toxic effects of methylmercury, an environmental contaminant found in fish [29,30]. Therefore, the consumption of fish, especially oily fish, on a regular basis is recommended for cardiovascular health [8,31]. Moreover, according to the latest scientific evidence, omega-3 fatty acid supplements are not useful, thus are not recommended for the prevention of CVD [32,33]. However, some large predatory fish, such as shark, sword-fish, tilefish, king mackerel, and bigeye tuna, have high levels of methylmercury, which may have neurotoxic effects in the fetus and reduce cognition in young children; hence, it is recommended to consume an optimal amount (1–2 servings a week) of fish so that the benefits can outweigh the risks, particularly for pregnant women and children [8].
The results of this study disclose that the blood lipid benefits were strongest for oily fish, such as mackerel, pacific saury, and eel, compared to leaner fish, including yellow croaker, flat fish, and hair tail. Though all kinds of fish are highly nutritious due to high contents of protein, fat soluble vitamins, and minerals, for preventing dyslipidemia and promoting cardiovascular health, regular consumption of oily fish is recommended.
This study had several limitations. First, we could not include all types of oily fish owing to predetermined lists of food items in the FFQ. Second, owing to the lack of an omega-3 fatty acids database, the intake of omega-3 fatty acids from fish could not be considered a covariable. Third, the effects of methylmercury on fish cannot be excluded, since methylmercury may attenuate the health benefits of omega-3 fatty acids [34]. However, the current evidence suggests that the benefits of consuming 1 to 2 servings of fish per week outweigh the risks, especially if a variety of seafood are consumed [8]. Further studies are required to examine the comprehensive effects of omega-3 fatty acids and methylmercury in fish on the risk of dyslipidemia.
Despite these limitations, this is the first prospective study to examine the association between oily fish consumption and the risk of dyslipidemia in the Korean population. Because of the prospective study design, we examined the causal association between oily fish consumption and the risk of dyslipidemia. The strengths of the present study using the HEXA-G sample include the relative homogeneity of the cohort, allowing for increased internal validity. Furthermore, we used a validated FFQ, which ensured standardized protocols to obtain information regarding the participants’ long-term dietary intake.
5. Conclusions
Increased oily fish consumption is significantly associated with a lower risk of hypertriglyceridemia after adjusting for potential confounders in the general Korean population. The present results suggest that oily fish consumption potentially protects against dyslipidemia and related chronic diseases among Korean adults. Future randomized clinical trials or prospective studies are needed to further analyze these findings in the Korean or other Asian populations.
Acknowledgments
We acknowledge the participants and all members of the HEXA Study Group.
Author Contributions
Conceptualization, S.S.; formal analysis, S.-A.K.; writing—original draft preparation, S.-A.K.; writing—review and editing, S.S., J.-k.L., and D.K.; supervision, S.S.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Orho-Melander M. Genetics of coronary heart disease: Towards causal mechanisms, novel drug targets and more personalized prevention. J. Intern. Med. 2015;278:433–446. doi: 10.1111/joim.12407. [DOI] [PubMed] [Google Scholar]
- 2.National Cholesterol Education Program Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. doi: 10.1161/circ.106.25.3143. [DOI] [PubMed] [Google Scholar]
- 3.Tóth P.P., Potter D., Ming E.E. Prevalence of lipid abnormalities in the united states: The national health and nutrition examination survey 2003–2006. J. Clin. Lipidol. 2012;6:325–330. doi: 10.1016/j.jacl.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Lee M.H., Kim H.C., Ahn S.V., Hur N.W., Choi D.P., Park C.G., Suh I. Prevalence of dyslipidemia among Korean adults: Korea national health and nutrition survey 1998–2005. Diabetes Metab. J. 2012;36:43–55. doi: 10.4093/dmj.2012.36.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ference B.A., Ginsberg H.N., Graham I., Ray K.K., Packard C.J., Bruckert E., Watts G.F., Boren J., Fazio S., Horton J.D., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordestgaard B.G., Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 7.Ooi E.M., Ng T.W., Watts G.F., Barrett P.H. Dietary fatty acids and lipoprotein metabolism: New insights and updates. Curr. Opin. Lipidol. 2013;24:192–197. doi: 10.1097/MOL.0b013e3283613ba2. [DOI] [PubMed] [Google Scholar]
- 8.Rimm E.B., Appel L.J., Chiuve S.E., Djoussé L., Engler M.B., Kris-Etherton P.M., Mozaffarian D., Siscovick D.S., Lichtenstein A.H. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: A science advisory from the American Heart Association. Circulation. 2018;138:e35–e47. doi: 10.1161/CIR.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mozaffarian D., Wu J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 10.Ebbesson S.O., Tejero M.E., Nobmann E.D., Lopez-Alvarenga J.C., Ebbesson L., Romenesko T., Carter E.A., Resnick H.E., Devereux R.B., MacCluer J.W., et al. Fatty acid consumption and metabolic syndrome components: The gocadan study. J. Cardiometab. Syndr. 2007;2:244–249. doi: 10.1111/j.1559-4564.2007.07393.x. [DOI] [PubMed] [Google Scholar]
- 11.Xun P., Hou N., Daviglus M., Liu K., Morris J.S., Shikany J.M., Sidney S., Jacobs D.R., He K. Fish oil, selenium and mercury in relation to incidence of hypertension: A 20-year follow-up study. J. Intern. Med. 2011;270:175–186. doi: 10.1111/j.1365-2796.2010.02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S., Song Y., Ford E.S., Manson J.E., Buring J.E., Ridker P.M. Dietary calcium, vitamin d, and the prevalence of metabolic syndrome in middle-aged and older U.S. Women. Diabetes Care. 2005;28:2926–2932. doi: 10.2337/diacare.28.12.2926. [DOI] [PubMed] [Google Scholar]
- 13.Wang L., Manson J.E., Buring J.E., Lee I.M., Sesso H.D. Dietary intake of dairy products, calcium, and vitamin d and the risk of hypertension in middle-aged and older women. Hypertension. 2008;51:1073–1079. doi: 10.1161/HYPERTENSIONAHA.107.107821. [DOI] [PubMed] [Google Scholar]
- 14.Korea Rural Economic Institute 2013 Food Balance Sheet. [(accessed on 20 April 2016)]; Available online: https://www.krei.re.kr/web/www/23?p_p_id=EXT_BBS&p_p_lifecycle=0&p_p_state=normal&p_p_mode=view&_EXT_BBS_struts_action=%2Fext%2Fbbs%2Fview_message&_EXT_BBS_messageId=404443.
- 15.Health Examinees Study Group The Health Examinees (HEXA) study: Rationale, study design and baseline characteristics. Asian Pac. J. Cancer Prev. APJCP. 2015;16:1591. doi: 10.7314/APJCP.2015.16.4.1591. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y., Han B.-G., Group K. Cohort profile: The Korean genome and epidemiology study (KoGES) consortium. Int. J. Epidemiol. 2016;46:e20. doi: 10.1093/ije/dyv316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin S., Lee H.-W., Kim C., Lim J., Lee J.-K., Lee S.-A., Kang D. Egg consumption and risk of metabolic syndrome in Korean adults: Results from the Health Examinees Study. Nutrients. 2017;9:687. doi: 10.3390/nu9070687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Obesity Task Force. World Health Organization . The Asian-Pacific Perspective: Redefining Obesity and its Treatment. Health Communications Australia; Melbourne, Australia: 2000. [Google Scholar]
- 19.Wang F., Wang Y., Zhu Y., Liu X., Xia H., Yang X., Sun G. Treatment for 6 months with fish oil-derived n-3 polyunsaturated fatty acids has neutral effects on glycemic control but improves dyslipidemia in type 2 diabetic patients with abdominal obesity: A randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2017;56:2415–2422. doi: 10.1007/s00394-016-1352-4. [DOI] [PubMed] [Google Scholar]
- 20.Petersen M., Pedersen H., Major-Pedersen A., Jensen T., Marckmann P. Effect of fish oil versus corn oil supplementation on LDL and HDL subclasses in type 2 diabetic patients. Diabetes Care. 2002;25:1704–1708. doi: 10.2337/diacare.25.10.1704. [DOI] [PubMed] [Google Scholar]
- 21.Harris W.S., Connor W.E., Illingworth D.R., Rothrock D.W., Foster D.M. Effects of fish oil on VLDL triglyceride kinetics in humans. J. Lipid Res. 1990;31:1549–1558. [PubMed] [Google Scholar]
- 22.Goldberg R.B., Sabharwal A.K. Fish oil in the treatment of dyslipidemia. Curr. Opin. Endocrinol. Diabetes Obes. 2008;15:167–174. doi: 10.1097/MED.0b013e3282f76728. [DOI] [PubMed] [Google Scholar]
- 23.Klop B., Elte J., Cabezas M. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients. 2013;5:1218–1240. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevenson J.C., Crook D., Godsland I.F. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis. 1993;98:83–90. doi: 10.1016/0021-9150(93)90225-J. [DOI] [PubMed] [Google Scholar]
- 25.Carpentier Y.A., Portois L., Malaisse W.J. n−3 Fatty acids and the metabolic syndrome. Am. J. Clin. Nutr. 2006;83:1499S–1504S. doi: 10.1093/ajcn/83.6.1499S. [DOI] [PubMed] [Google Scholar]
- 26.Eslick G.D., Howe P.R., Smith C., Priest R., Bensoussan A. Benefits of fish oil supplementation in hyperlipidemia: A systematic review and meta-analysis. Int. J. Cardiol. 2009;136:4–16. doi: 10.1016/j.ijcard.2008.03.092. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J., Wang C., Li L., Man Q., Song P., Meng L., Du Z.-Y., Frøyland L. Inclusion of Atlantic salmon in the Chinese diet reduces cardiovascular disease risk markers in dyslipidemic adult men. Nutr. Res. 2010;30:447–454. doi: 10.1016/j.nutres.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J., Wang C., Li L., Man Q., Meng L., Song P., Frøyland L., Du Z.-Y. Dietary inclusion of salmon, herring and pompano as oily fish reduces CVD risk markers in dyslipidaemic middle-aged and elderly Chinese women. Br. J. Nutr. 2012;108:1455–1465. doi: 10.1017/S0007114511006866. [DOI] [PubMed] [Google Scholar]
- 29.Ganther H.E. Modification of methylmercury toxicity and metabolism by selenium and vitamin E: Possible mechanisms. Environ. Health Perspect. 1978;25:71–76. doi: 10.1289/ehp.782571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mozaffarian D., Rimm E.B. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 31.He K. Fish, long-chain omega-3 polyunsaturated fatty acids and prevention of cardiovascular disease—eat fish or take fish oil supplement? Prog. Cardiovasc. Dis. 2009;52:95–114. doi: 10.1016/j.pcad.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Aung T., Halsey J., Kromhout D., Gerstein H.C., Marchioli R., Tavazzi L., Geleijnse J.M., Rauch B., Ness A., Galan P., et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: Meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3:225–233. doi: 10.1001/jamacardio.2017.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdelhamid A.S., Brown T.J., Brainard J.S., Biswas P., Thorpe G.C., Moore H.J., Deane K.H.O., AlAbdulghafoor F.K., Summerbell C.D., Worthington H.V., et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018;7:CD003177. doi: 10.1002/14651858.CD003177.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Ascoli T.A., Mursu J., Voutilainen S., Kauhanen J., Tuomainen T.P., Virtanen J.K. Association between serum long-chain omega-3 polyunsaturated fatty acids and cognitive performance in elderly men and women: The Kuopio Ischaemic Heart Disease Risk Factor Study. Eur. J. Clin. Nutr. 2016;70:970. doi: 10.1038/ejcn.2016.59. [DOI] [PubMed] [Google Scholar]