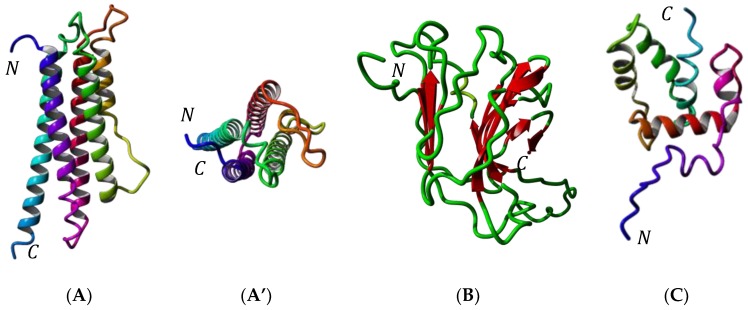
Figure 3.
(A) Ribbon diagram of apolipophorin-III of Tenebrio molitor (sagital view). Alpha-helices numbered α1–α5 are differently colored. N and C correspond to the N- and C-terminus of the polypeptide chain, respectively. (A’) Ribbon diagram of apolipophorin-III of T. molitor: upper view showing the arrangemernt of α-helices around a central hydrophobic pocket. (B) Ribbon diagram of the larval cuticlar protein (LCP) of T. molitor, built up from the association of two antiparallel bundles of β-sheet in a β-sandwich structure. (C) Ribbon diagram of the 12 kDa hemolymph protein (12 kDa HLP) of T. molitor, built up from the association of six α-helices around a central hydrophobic pocket. Alpha-helices are differently colored.