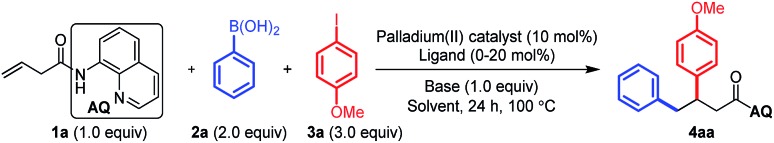
Table 1. Optimization of the directed three-component syn-vicinal-diarylation of alkene 1a with phenylboronic acids 2a and 4-methoxyphenyl iodide 3a a .
| ||||
| Entry | PdII cat. | Base | Solvent | Yield b [%] |
| 1 | Pd(OAc)2 | K2CO3 | HFIP (2 mL) | NR |
| 2 | Pd(OAc)2 | Na2CO3 | DCM/H2O/MeCN (1 : 0.2 : 0.1) | 54 |
| 3 | PdCl2 | Na2CO3 | DCM/H2O/MeCN (1 : 0.2 : 0.1) | 44 |
| 4 | Pd(PhCN)2Cl2 | Na2CO3 | DCM/H2O/MeCN (1 : 0.2 : 0.1) | 69 |
| 5 | (–)-SparteinePdCl2 | Na2CO3 | DCM/H2O/MeCN (1 : 0.2 : 0.1) | 71 |
| 6 | (–)-SparteinePd(OAc)2 | Na2CO3 | DCM/H2O/MeCN (1 : 0.2 : 0.1) | 6 |
| 7 | (Bipy)PdCl2 | Na2CO3 | DCM/H2O/MeCN (1 : 0.2 : 0.1) | 60 |
| 8 | PddppfCl2 | Na2CO3 | DCM/H2O/MeCN (1 : 0.2 : 0.1) | 11 |
| 9 | (–)-SparteinePdCl2 | K2CO3 | DCM/H2O/MeCN (1 : 0.2 : 0.1) | 66 |
| 10 | (–)-SparteinePdCl2 | Cs2CO3 | DCM/H2O/MeCN (1 : 0.2 : 0.1) | 44 |
| 11 | (–)-SparteinePdCl2 | K3PO4 | DCM/H2O/MeCN (1 : 0.2 : 0.1) | 53 |
| 12 c | (–)-SparteinePdCl 2 | Na 2 CO 3 | DCM/H 2 O/MeCN (1 : 0.2 : 0.1) | 83 |
| 13 c | (–)-SparteinePdCl2 | Na2CO3 | DCM/H2O/MeCN (1 : 0.1 : 0.1) | 72 |
| 14 c | (–)-SparteinePdCl2 | Na2CO3 | DCM/H2O (1 : 0.2) | 77 |
| 15 c | (–)-SparteinePdCl2 | Na2CO3 | DCM/CH3CN (1 : 0.1) | 14 |
| 16 c | (–)-SparteinePdCl2 | Na2CO3 | DCM (2 mL) | 18 |
| 17 c | (–)-SparteinePdCl2 | Na2CO3 | HFIP (2 mL) | NR |
| 18 c d | (–)-SparteinePdCl2 | Na2CO3 | DCM/H2O/MeCN (1 : 0.2 : 0.1) | 81 |
| 19 c e | (–)-SparteinePdCl2 | Na2CO3 | DCM/H2O/MeCN (1 : 0.2 : 0.1) | 35 |
aReactions were carried out by using PdII catalyst (10 mol%), ligand (0–20 mol%), base (0.3 mmol, 1.0 equiv.), 1a (0.3 mmol, 1.0 equiv.), phenylboronic acid 2a (2.0 equiv.), and 4-methoxyphenyl iodide 3a (3.0 equiv.) in the mixture of DCM/H2O/CH3CN (2 mL : 0.4 mL : 0.2 mL) for 24 h at 100 °C under a N2 atmosphere.
bIsolated yields.
c(–)-Sparteine (20 mol%) was added.
d(PhBO)3 instead of 2a.
ePhBNep instead of 2a.
