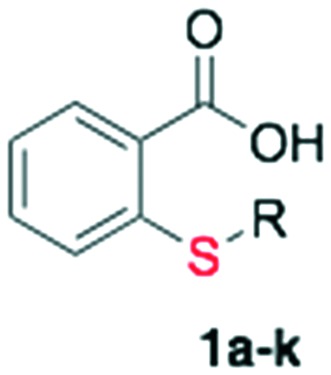
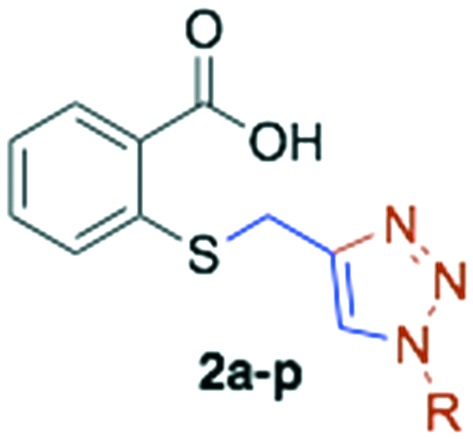
Table 2. In vitro antimalarial activity and yield of alkylated thiosalicylic acid derivatives and of 1,2,3-triazolyl thiosalicylic acid derivatives.
| Entry | Cmpd | R | Yield (%) | P. falciparum a IC50 μM | Z value | R 2 | |
| 1 | 1 | Propargyl | 81 | >200 |
|
||
| 2 | 1a | Cyclohexyl | 87 | >200 | |||
| 3 | 1b | Benzyl | 73 | >200 | |||
| 4 | 1c | 3-Phenyl-propyl | 75 | >200 | |||
| 5 | 1d | Cinnamyl | 68 | >200 | |||
| 6 | 1e | Octyl | 83 | >200 | |||
| 7 | 1f | Decyl | 81 | >200 | |||
| 8 | 1g | CH2CH2CO2CH2CH3 | 78 | >200 | |||
| 9 | 1h | Prenyl | 72 | >200 | |||
| 10 | 1i | Geranyl | 88 | >200 | |||
| 11 | 1j | Farnesyl (Salirasib) | 79 | 20.34 ± 5.52 | 0.93 ± 0.06 | 0.95 ± 0.02 | |
| 12 | 1k | Phytyl | 85 | 13.40 ± 1.53 | 0.93 ± 0.06 | 0.98 ± 0.02 | |
| 13 | 2a | Cyclohexyl | 84 | >200 |
|
||
| 14 | 2b | Benzyl | 80 | >200 | |||
| 15 | 2c | 3-Phenyl-propyl | 88 | >200 | |||
| 16 | 2d | Cinnamyl | 86 | 65.33 ± 10.71 | 0.89 ± 0.09 | 0.87 ± 0.01 | |
| 17 | 2e | Octyl | 78 | >200 | |||
| 18 | 2f | Decyl | 88 | >200 | |||
| 19 | 2g | CH2CH2CO2CH2CH3 | 91 | >200 | |||
| 20 | 2h | Prenyl | 89 | >200 | |||
| 21 | 2i | Geranyl | 84 | >200 | |||
| 22 | 2j | Farnesyl | 90 | 25.97 ± 1.32 | 0.94 ± 0.06 | 0.96 ± 0.02 | |
| 23 | 2k | Phytyl | 83 | 9.75 ± 1.97 | 0.92 ± 0.06 | 0.98 ± 0.01 | |
| 24 | 2l | CH2CO2CH2CH3 | 92 | >200 | |||
| 25 | 2m | Cetyl | 81 | 14.44 ± 5.43 | 0.93 ± 0.06 | 0.97 ± 0.01 | |
| 26 | 2n | Tridecanyl | 55 | 19.83 ± 5.14 | 0.88 ± 0.08 | 0.97 ± 0.02 | |
| 27 | 2o | 2-Phenyl-ethyl | 94 | >200 | |||
| 28 | 2p | 2-Naphthylmethyl | 33 | 20.54 ± 5.45 | 0.92 ± 0.06 | 0.96 ± 0.04 | |
| CQ | 8.15 ± 1.83 nM | 0.75 ± 0.18 | 0.96 ± 0.02 | ||||
| ART | 1.64 ± 0.08 nM | 0.86 ± 0.11 | 0.96 ± 0.03 |
aEvaluated by the NanoLuc luciferase method.
