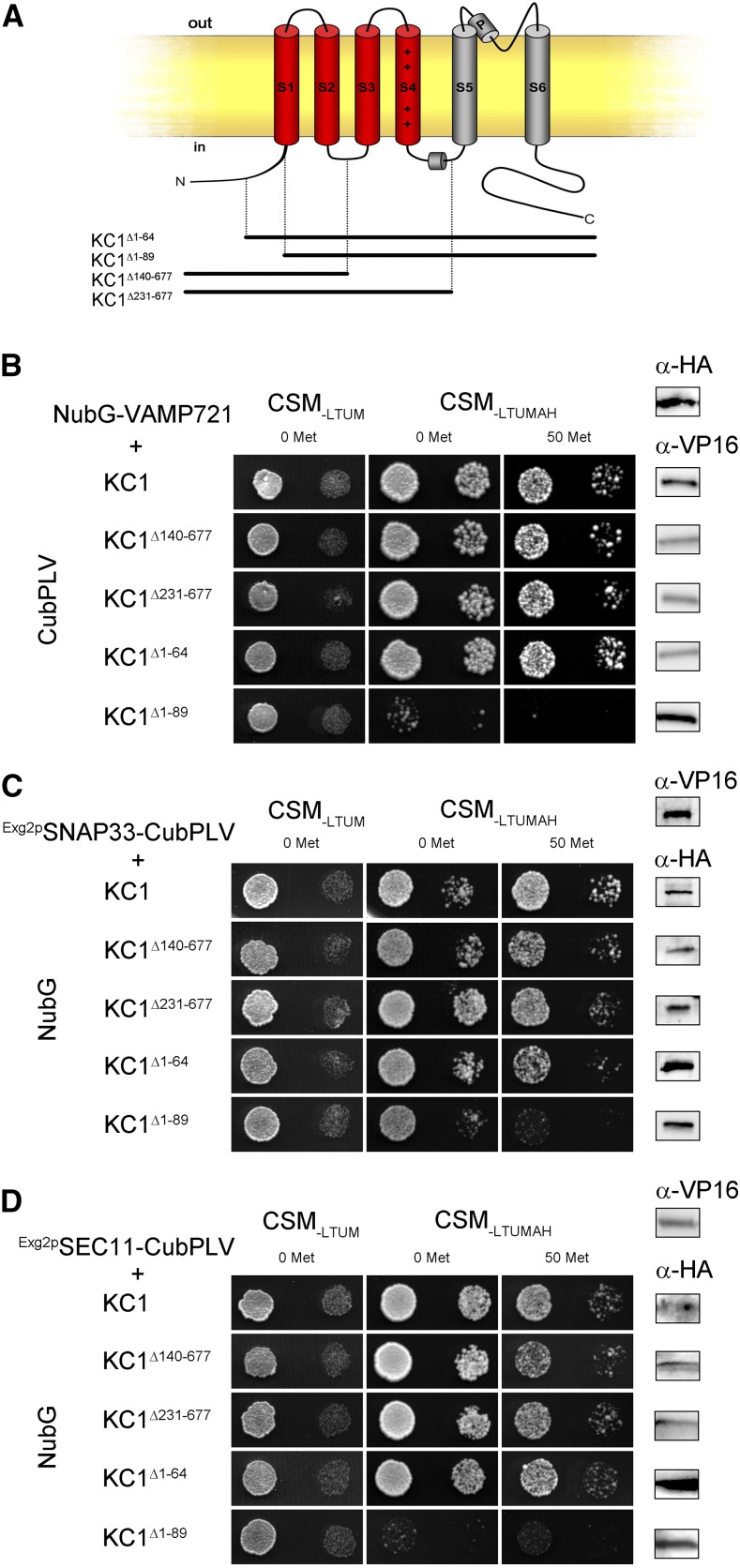
Figure 1.
KC1 K+ channel interacts with VAMP721, SNAP33, and SEC11 via a cytosolic N-terminal region of its voltage sensor domain. A, Schematic of Kv channel structure with the VSD comprising α-helices S1 to S4 identified in red and the pore-lining α-helices S5 and S6 in gray (adapted from Grefen et al. [2015]). Segments expressed for the KC1 deletions are indicated below. B to d, Yeast mbSUS assay for interaction of the VAMP721, SNAP33, and SEC11 fusions with KC1 and its deletion constructs (A) as bait with Y-Cub fusions. VAMP721, SNAP33, and SEC11 served as NubG-X prey fusions and SNAP33 and SEC11 were anchored via the GPI signal peptide (Zhang et al., 2018). Positive and negative controls are included in Supplemental Figure S1. Similar results were obtained in three independent experiments. Growth on CSM-LTUM was used to verify the presence of both bait and prey expression. CSM-LTUMAH was used to verify Ade- and His-independent growth of the yeast diploids. The addition of 50 μm Met to CSM-LTUMAH suppressed bait expression as a test for interaction specificity. Yeast was dropped at 1.0 and 0.1 OD600 in each case. Incubation time was 24 h for the CSM-LTUM plate and 72 h for CSM-LTUMAH plates. Immunoblot analysis (5 μg total protein/lane) of the haploid yeast used in mating (right) used the αHA antibody for the prey fusions and the αVP16 antibody for the bait fusions.