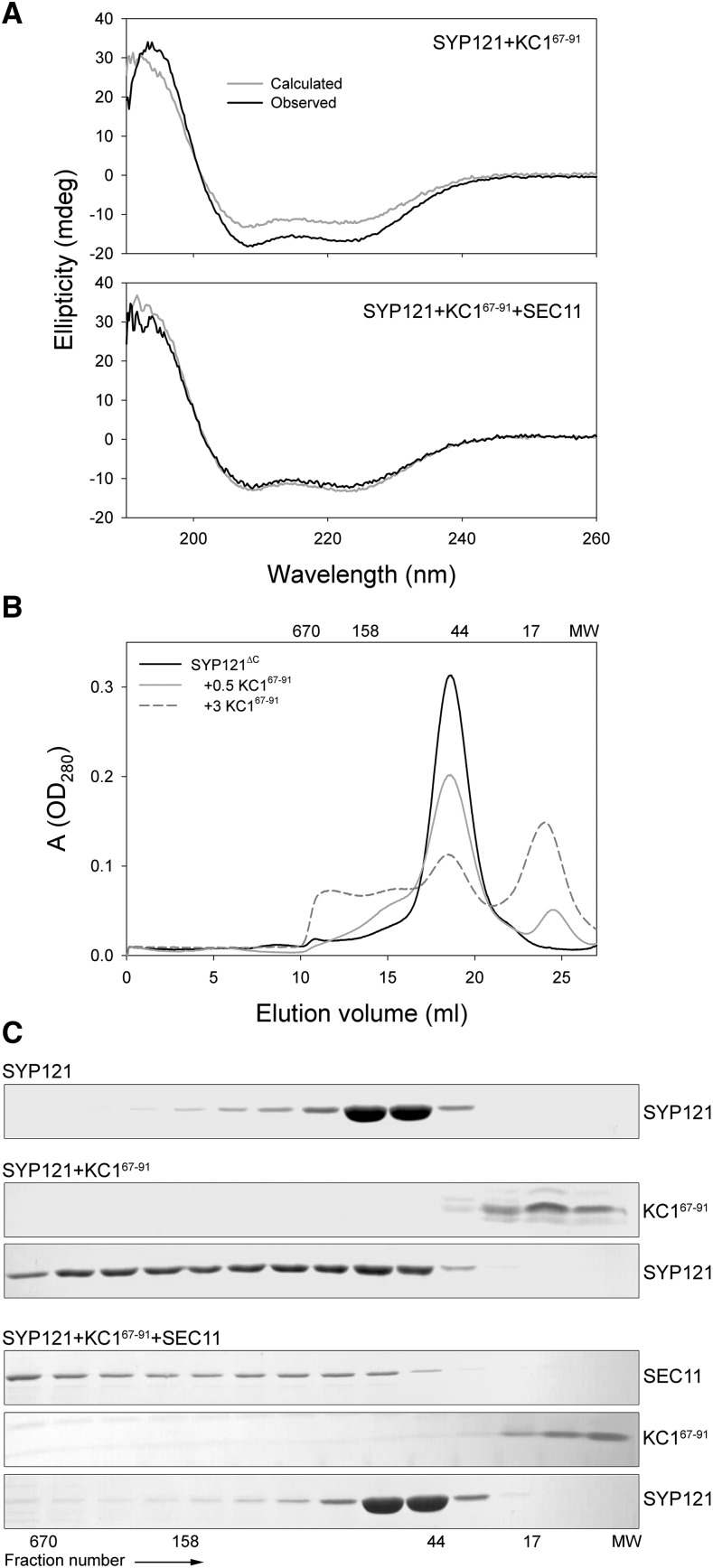
Figure 6.
The K+ channel binding domain alters SYP121 structure to promote the open conformation. A, Far-UV CD spectra of KC167–91 with SYP121ΔC-2PA mixed and incubated overnight in equimolar ratio shows roughly a 24% increase in spectral peak amplitudes between 200 and 230 nm above the arithmetical sum of individual spectra for the two proteins (top). This shift in peak amplitudes is suppressed on coincubation with SEC11 (bottom). Data are from one of three independent experiments, all of which yielded equivalent results with a mean of 28 ± 5% in peak amplitude above the spectral sum. Similar results were obtained with KAT11–63 (see Supplemental Fig. S6). B, Size-exclusion chromatography of SYP121ΔC-2PA incubated overnight at 4°C alone and on coincubation with KC167–91. In the closed conformation, SYP121ΔC-2PA eluted as a single peak corresponding to a molecular weight around 48 kD. When mixed with increasing amounts of the channel peptide, this peak was reduced in favor of a higher-molecular weight shoulder and peak spanning to near 650 kD, consistent with transition to the Qa-SNARE open conformation and formation of multimers. Molecular weights of standards are indicated above the columns. C, SDS-PAGE analysis of the gel filtration eluate collected in 0.75-mL fractions for fractions 11 to 24 collected after overnight incubations of SYP121ΔC-2PA alone, with KC167–91, and with KC167–91 together with SEC11. Note the shift of the SYP121ΔC-2PA to higher-molecular weight fractions in the presence of the channel peptide and its suppression when SEC11 is included in the incubation mix. Molecular weights of standards are indicated below the columns.