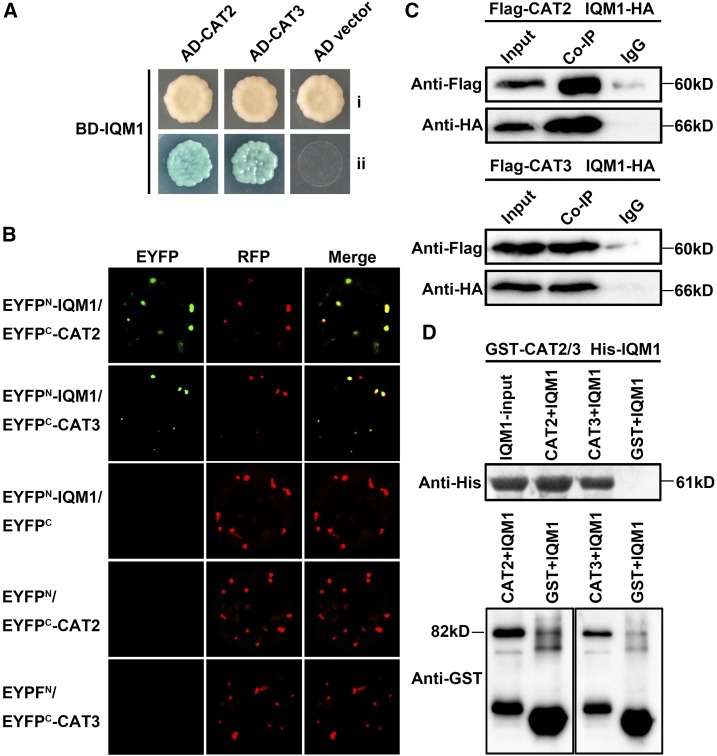
Figure 2.
IQM1 directly interacts with CAT2 in planta. A, IQM1 interacts with CAT2/3 in yeast cells. pGADT7-CAT2/3 and pGBKT7-IQM1 were cotransformed into AH109 yeast cells, which were grown on SD/-Trp-Leu (i) and SD/-Trp-Leu-His-Ade containing 20 µg mL−1 x-α-gal (ii) for 3 d. B, BiFC assays to detect the interaction between IQM1 and CAT2/3. nEYFP-IQM1 and cEYFP-CAT2/3 plasmids were cotransformed into protoplasts isolated from the leaves of 4-week-old Col-0 plants. After 16 h of incubation, the EYFP signals were observed using a laser confocal microscope. RFP-AtKAT1 was used as a colocalization marker protein. nEYFP-IQM1 and cEYFP empty vector or nEYFP empty vector and cEYFP-CAT2/3 were set as negative controls. C, Co-IP assay to verify the interaction of IQM1 with CAT2/3 in planta. Flag-CAT2/3 and IQM1-HA plasmids were cointroduced into protoplasts isolated from the leaves of 4-week-old Col-0 plants with polyethylene glycol (PEG)/CaCl2. Protein G Plus/Protein A-Agarose and anti-Flag antibody were added to the plant protein, and the precipitated proteins were separated by 12% (w/v) SDS-PAGE for immunoblotting with anti-Flag antibody and anti-HA antibody. Proteins mixed with beads and IgG were used as negative controls. D, In vitro pull-down assay to verify the direct interaction of IQM1 with CAT2/3. His-IQM1 and GST-CAT2/3 plasmids were separately transformed into the E. coli strain Rosetta. The recombinant fusion proteins were purified with Ni-NTA agarose and Glutathione Sepharose. Agarose-conjugated His-IQM1 was then washed with imidazole. All eluates were added to agarose-conjugated GST-CAT2/3. The precipitated proteins were separated by 12% (w/v) SDS-PAGE for immunoblotting with anti-His antibody and anti-GST antibody. His-IQM1 plus GST beads and purified GST were used as negative controls. All experiments were repeated at least three times.