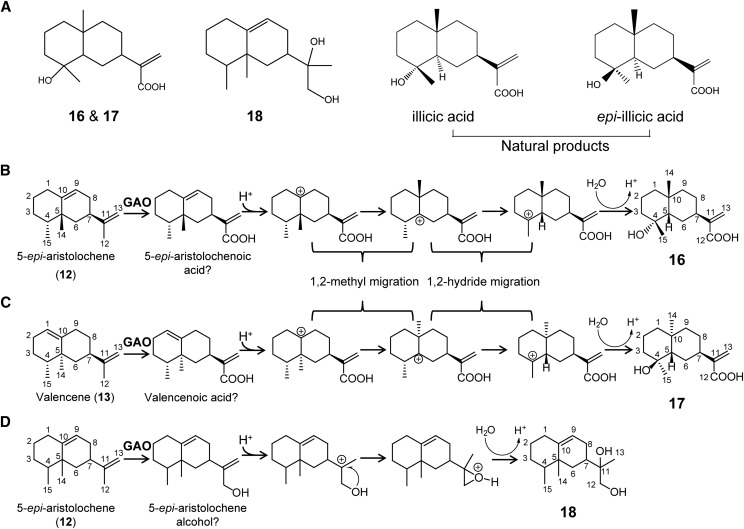
Figure 6.
Oxidations and rearrangements of 5-epi-aristolochene and valencene in yeast expressing GAOs. A, Structures of the compounds synthesized from transgenic yeasts and structures of natural products, illicic acid, and epi-illicic acid. B and C, Oxidations at C12 of 5-epi-aristolochene (B) and valencene (C) to the respective sesquiterpene acids. Skeletal rearrangements after protonations by acid are proposed to explain the occurrence of illicic acids. D, A proposed skeletal rearrangement of 5-epi-aristolochene alcohol to compound 18. Question marks indicate hypothetical compounds.