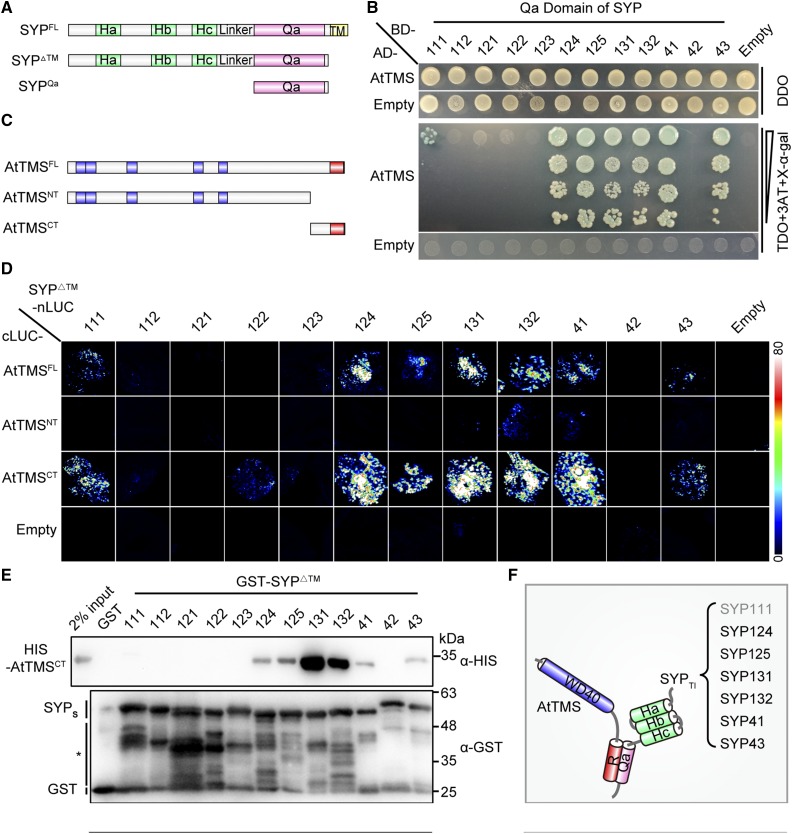
Figure 3.
The C terminus of AtTMS and the SNARE domain of SYPs mediate mutual binding between TMS-interacting SYP (SYPsTI) and AtTMS. A, Schematic presentation of truncations for SYP proteins. B, A yeast two-hybrid assay showed that AtTMS interacts with the Qa domain of SYP111/124/125/131/132/41/43. Transformed yeast cells able to grow and turn blue on triple dropout medium (-Leu-Trp-His; TDO) + 5 µm 3-aminotriazole (3AT) + 200 ng mL−1 5-bromo-4-chloro-3-indolyl-α-d-galactopyranoside (X-α-gal) indicates positive interactions. DDO, Double dropout medium (-Leu-Trp). C, Schematic representation of the size and position of each AtTMS truncation. The WD40 domain (blue) and R-SNARE motif (red) are indicated. D, SYP111/124/125/131/132/41/43ΔTM interact with both the AtTMSFL and the AtTMSCT in firefly LCI assays. Different pairs of the indicated plasmids were cotransformed in tobacco leaves. A pair of cLUC and nLUC empty vectors was used as negative controls (Empty). The luciferase signal was captured using a low-light cooled CCD camera, and the color scale at right shows the range of luminescence intensity. E, Only SYP111/124/125/131/132/41/43ΔTM can interact with the AtTMSCT. GST and each GST-SYPΔTM protein coupled to beads were incubated with His-tagged AtTMSCT. Bound proteins were detected using mouse anti-His antibody. The blot was stripped and reblotted with mouse anti-GST antibody to ensure the quality and coupling of the bait proteins. The asterisk indicates nonspecific or degraded protein bands. F, Diagram of the mode of interaction between AtTMS and SYPsTI. Gray text for SYP111 indicates a weak interaction.