Abstract
Objectives
We studied erythrocyte (RBC) caspase-3 activity and oxidative status in plasma and RBCs of 33 patients with type 2 diabetes at first clinical onset and 23 age-matched non-diabetes control subjects.
Methods
Caspase-3 activity was assayed during the life span of RBCs; lipid peroxides and total antioxidant capacity (TEAC) were assessed in plasma and RBCs as indicators of oxidative stress and non-enzymatic antioxidant defense; and superoxide dismutase, catalase, and glutathione peroxidase activity were measured in RBCs as enzymatic antioxidants.
Results
We found that, compared to controls, RBCs caspase-3 is activated early in type 2 diabetes (P < 0.05); TEAC and malondialdehyde increased in plasma of patients with early diabetes, even when hypertension and macroangiopathy were present (P < 0.01); and RBCs TEAC, malondialdehyde (P < 0.01), superoxide dismutase, and glutathione peroxidase (P < 0.05) exhibited similar behavior in patients with diabetes and hypertensive patients with diabetes.
Discussion
Increased antioxidant defense in plasma and RBCs of early type 2 diabetes patients is a potential mechanism that can overcome oxidative damage induced by reactive oxygen species overproduction, and occurs even in RBCs with a decreased life span. This observation could provide a possible explanation for the controversial effects of antioxidant supplementation in diabetes patients.
Keywords: Diabetes, Oxidative status, Erythrocytes, Caspase
Introduction
In type 2 diabetes, β-cell exposure to chronic hyperglycemia induces excess generation of reactive oxygen species (ROS).1 Increased oxidative stress deteriorates pancreatic β-cells and therefore essentially contributes to the pathogenesis of diabetes.2 Oxidative stress associated with type 2 diabetes mellitus may contribute to microvascular and macrovascular complications.3 Antioxidant systems involved in redox regulation of the cell are major tools for neutralizing oxidative stress and therefore protect the cells from oxidative stress-induced damage. It has been shown that an imbalanced redox mechanism may contribute to the pathogenesis of diabetes mellitus or diabetes complications.4,5 However, the antioxidant status of plasma in patients with type 2 diabetes remains controversial.6,7 The magnitude of oxidative stress and antioxidant response in type 2 diabetes differs in various cells and organs, and tissues with high metabolic demands, such as blood cells, are mainly targeted.8 Increased oxidative damage and decreased life span of red blood cells (RBCs) are reported in patients with late type 2 diabetes.9,10 It is well known that a number of enzymatic systems protect erythrocytes from the damage caused by excessive production of ROS. These systems include superoxide dismutase (SOD), which converts superoxide into hydrogen peroxide, together with glutathione peroxidase (GPX) and catalase (CAT), which convert hydrogen peroxide to water. Although these antioxidant enzymes in blood have been cited as markers of vascular injury in type 2 diabetes,11 conflicting data have been reported about their activities.12–14 Moreover, there are few data about the non-enzymatic antioxidant capacity of erythrocytes in patients with late type 2 diabetes.9,10 We therefore proposed to study erythrocyte caspase-3 activity and oxidative status in plasma and RBCs of patients with type 2 diabetes at first clinical onset.
Materials and methods
Subjects
Our study included 33 patients with type 2 diabetes at first clinical onset, randomly selected during routine examination. Twenty-three age-matched volunteers were recruited as controls from general practitioner files. Type 2 diabetes patients were recruited according to American Diabetes Association criteria,15 and were examined for identification of possible chronic specific complications of diabetes, including microangiopathy, macroangiopathy, and hypertension. None of the subjects in the study had known hematological diseases. Informed consent was obtained from all participants before the screening visit. The study was conducted at the ‘N C Paulescu’ National Institute for Diabetes, Nutrition and Metabolic Diseases, Bucharest, Romania and was approved by the Ethical Committee of the Institute. Subject characteristics are presented in Table 1.
Table 1.
Subject characteristics*
| Controls | Type 2 DM (n = 33) | ||||
|---|---|---|---|---|---|
| No complications | HBP | Macroangiopathy | P vs. controls | ||
| n (%) | 23 (100) | 13 (39.4) | 13 (39.4) | 7 (21.2) | |
| Age (years) | 59.6 ± 2.4 | 54.9 ± 2.9 | 52.8 ± 4.0 | 62.8 ± 2.6 | NS |
| BMI (kg/m2) | 27.3 ± 0.3 | 26.3 ± 0.9 | 28.7 ± 0.7 | 28.9 ± 0.7 | NS |
| HbA1c (%) | 5.5 ± 0.05 | 8.3 ± 0.5 | 8.5 ± 0.5 | 9.1 ± 0.6 | <0.001 |
| SBP (mmHg) | 132.9 ± 1.2 | 130.5 ± 2.8 | 171.9 ± 2.6 | 155.7 ± 2.6 | <0.001 |
| DBP (mmHg) | 78.2 ± 1.1 | 82.4 ± 2.1 | 91.2 ± 2.6 | 95.1 ± 2.6 | <0.001 |
*Values are mean ± SE. P = NS for SBP and DBP between controls and T2DM group.
DM, diabetes mellitus; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HBP, high blood pressure.
Sample processing
To evaluate the oxidative stress parameters, blood samples (10 ml), collected after overnight fasting, were placed into 2-ml vacutainer tubes containing 34 IU lithium heparin. After centrifugation (548 g for 15 minutes at 4°C), the plasma was retained on ice for the assay of lipid peroxides (malondialdehyde–thiobarbituric acid (MDA–TBA) adduct) and total antioxidant activity (TEAC). The remaining erythrocytes (RBCs) were washed three times with 0.9% NaCl and lyzed in ultra-pure water. The red cell stroma was removed by centrifugation (10 minutes at 4°C), and the clarified supernate was kept on ice and diluted as required. Lipid peroxides (MDA–TBA adduct), TEAC, GPX, CAT, SOD, and caspase activity were assayed. Reagents and ultra-pure water were treated with Chelex 100 (Merck, Darmstadt, Germany) to bind transitional metals. Hemoglobin (Hb) content of RBCs was measured by using Drabkin reagent. All reagents were of pure analytical quality and were purchased from Sigma-Aldrich Chemie (Steinheim, Germany), unless otherwise indicated. All assays were performed by spectrophotometry on a Perkin-Elmer Lambda EZ 210 (Perkin-Elmer, Boston, MA, USA) and were carried out on duplicate samples.
Hemoglobin content
The Hb content of RBCs was measured by using Drabkin reagent (Sigma). Briefly, 250 µl of reaction volume containing 5 µl of sample were first incubated (20 minutes, RT), and then the absorbance was read at 540 nm by spectrophotometry.
Lipid peroxide test
Blood samples (50 µl plasma or 25 µl RBCs lysate diluted 1:5) were mixed with 25 or 10 µl, respectively, of 2% butylated hydroxytoluene (to prevent lipid oxidation during the assay); 750 or 500 µl, respectively, of TBA (final concentration 0.67%); and 0.5 ml trichloracetic acid (final concentration 20%), and incubated at 100°C for 1 hour. The reaction was stopped by cooling the samples under tap water, the pink MDA–TBA adduct was extracted in n-butanol for plasma samples only, and the absorbance of the organic layer was read at 532 nm after centrifugation at 8000 g for 15 minutes at 4°C. The concentration of lipid peroxidation products was calculated as MDA equivalents,16 and was expressed as nanomoles of MDA equivalents per milliliter of plasma or micromoles of MDA equivalents per gram of Hb. A 1,1,3,3-tetramethoxypropane standard curve was prepared for each run.
Total antioxidant activity
Total antioxidant activity was determined based on the 6-hydroxy-2,5,7,8-tetramethylchroman-2 carboxylic acid (Trolox, Sigma Aldrich Chemie, Munich, Germany) equivalent antioxidant capacity assay (TEAC) developed by Miller et al.17 with modifications.18 The TEAC assay measures the relative abilities of antioxidants to scavenge the 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation (ABTSx+), compared with the antioxidant potency of standard amounts of Trolox, the water-soluble vitamin E analog. The ABTS radical was generated from interaction between ABTS and potassium persulfate. Plasma or erythrocytes samples (10 µl) were mixed with 1 ml of 47 µM ABTSx+ and incubated for 1 minute at 30°C. Optical density (absorbance) was read at 734 nm against 5 mM phosphate buffered saline (pH 7.4). The percentage inhibition of absorbance, which is directly proportional to the antioxidant activity of the sample, was calculated. The assay was calibrated against a calibration curve with Trolox as standard. Plasma TEAC was expressed as millimoles per liter of Trolox, and RBC TEAC was expressed as micromoles of Trolox per gram Hb.
GPX activity
GPX (EC. 1.11.1.9) activity was determined on erythrocyte lysates using tert-butyl hydroperoxide as the substrate. Oxidized glutathione produced by GPX and peroxide was reduced by glutathione reductase and NADPH, with the decrease in concentration of NADPH recorded at 340 nm. Assay kinetics was calculated using a molar absorptivity of 6220/ M/cm at 340 nm.19
SOD activity
SOD (EC. 1.15.1.1) activity was determined as described by Marklund et al.20 The method is based on the ability of SOD to inhibit pyrogallol autoxidation. CuZnSOD from erythrocytes is extracted with an extraction reagent containing methanol:chloroform 62.7:37.5 (v/v) stored at 2–8°C. After the addition of the extraction reagent, the mixture is vortexed for 30 seconds and centrifuged for 5 minutes at 3000 g and 4°C. The upper aqueous layer contains the enzyme. The rate of autoxidation of 2 mM pyrogallol in the reaction buffer (TRIS-cacodilic acid 50 mM, pH = 8.2, containing 1 mM DTPA) – with and without enzyme – was taken from the increase in absorbance at 420 nm. A unit of the enzyme is generally defined as the amount of enzyme that inhibits the reaction by 50%. Results are corrected for the dilution and expressed relative to Hb content.
Erythrocyte CAT activity
CAT (EC. 1.11.1.6) activity was measured using the method described by Aebi.21 The erythrocyte lysate was diluted in 0.05 M potassium phosphate buffer (pH = 7), and the reaction was started by adding 10 mM hydrogen peroxide. Decrease in absorbance at 240 nm was measured for 30 seconds. Enzyme activity was calculated as a function of the rate constant of the first-order reaction (k), and was expressed as k per gram of Hb.
RBC caspase-3 activity
Caspase-3 activity was assayed by Abcam's Caspase 3 assay kit (colorimetric; Abcam, Cambridge, UK), according to the manufacturer's recommendations. Briefly, RBC lysate (diluted 1:5) containing 100 µg of protein was mixed with an equal volume (50 µl) of reaction buffer containing 10 mM DDT and 5 µl of substrate DEVD-pNA (200 µM final concentration). Samples were then incubated at 37°C for 1.5 hours. The absorbance was read at 400 nm against a negative control (sample without substrate).
HbA1c
HbA1c was assayed by standardized immunoturbidimetry (Cobas Integra®, Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions.
Statistical analysis
Data analysis was performed using the GraphPad InStat software package (GraphPad Software, La Jolla, CA, USA). Differences between groups were computed using analysis of variance parametric (Tukey's) or nonparametric (Kruskal–Wallis) tests. The strength of association between pairs of variables was assessed by Pearson's correlation coefficient or the Spearman rank correlation. A value of P < 0.05 was considered statistically significant.
Results
Among diabetes patients, 13 (39.4%) had no chronic complications. Hypertension was found in 13 (39.4%) cases, and macrovascular complications were diagnosed in seven (21.2%) diabetes patients. We first evaluated the levels of ROS by detection of lipid peroxide concentrations in blood samples of all study subjects. As shown in Figs. 1B and 2B, ROS overproduction was observed both in plasma and RBCs of early type 2 diabetes patients. MDA–TBA adducts significantly increased in plasma of early diabetes patients (Fig. 1B), even when hypertension and macroangiopathy were documented (P < 0.01 vs. controls; P = NS between diabetes groups). Erythrocyte lipid peroxides exhibit similar behavior (Fig. 2B), especially in diabetes patients and in hypertensive patients with diabetes (P < 0.001 vs. controls; P = NS between diabetes patients). Lipid peroxidation is a well-established mechanism of ROS-mediated oxidative damage, and the measurement of MDA–TBA adduct is a convenient method for its quantification.22 Having in mind the synergistic effect of antioxidants in human plasma,23 we next estimated antioxidant status by measuring total antioxidant capacity of the plasma in our subjects (Fig. 1A). We observed a significant increase in TEAC in diabetes patients (P < 0.01 vs. controls; P = NS between diabetes groups). The ability of free radicals to permeate biological membrane is well known,24–26 as is the importance of erythrocytes as a defense system of the blood against oxidative stress.27 Hence, we investigated non-enzymatic antioxidant activity in RBCs of all subjects enrolled. The TEAC assay is validated as a standard method for measuring total antioxidant capacity of a biological sample.28 We were therefore able to show a significant increase in TEAC in the RBCs of diabetes patients compared with that of non-diabetes subjects (Fig. 2A), especially when hypertension was documented (P < 0.01 and <0.001, respectively; P = NS between diabetes groups). We then analysed enzymatic antioxidants by measuring the activity of SOD, CAT, and GPX in RBCs of our subjects (Fig. 3). However, a significant increase was seen for SOD and GPX, but not for CAT, in type 2 diabetes patients. The effect appeared more evident for GPX (P < 0.05, P < 0.001, and P < 0.01, respectively, vs. controls; P = NS between diabetes groups) than for SOD (P < 0.05 vs. controls in diabetes and in diabetes and hypertensive groups). Finally, taking into account that RBCs are subject to increased oxidative stress and a shortened life span in late type 2 diabetes,10 we also investigated caspase-3 activity in our patients. We were able to show that caspase-3 activation occurs early in diabetes (Fig. 4), even when hypertension and macroangipathy were diagnosed (P < 0.05 vs. controls; P = NS between diabetes groups). We therefore report increased antioxidant activity in blood samples of early type 2 diabetes patients, which occurs even in RBCs with a decreased life span. Our results may represent a potential mechanism that can overcome oxidative damage induced by ROS overproduction in early type 2 diabetes, and could provide a possible explanation for the controversial effects of antioxidant supplementation in diabetes patients.
Figure 1.
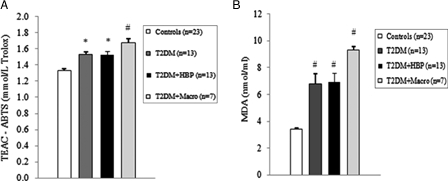
Oxidative stress and total antioxidant status of plasma increases in early type 2 diabetes patients. Plasma from type 2 diabetes patients at first clinical onset and from non-diabetes age-matched subjects was evaluated for Trolox equivalent antioxidant capacity (TEAC) (A) and malondialdehyde–thiobarbituric acid (MDA–TBA) adducts (B). Values are mean ± SE. *P < 0.01, #P < 0.001 vs. controls (analysis of variance Kruskal–Wallis test).
Figure 2.
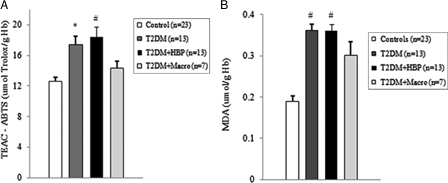
Oxidative stress and total antioxidant status of RBCs is increased in type 2 diabetes patients at first clinical onset. Red blood cells (RBCs) from early type 2 diabetes patients and from non-diabetes age-matched subjects were evaluated for TEAC (A) and MDA–TBA adducts (B). Values are mean ± SE. *P < 0.01, #P < 0.001 vs. controls (analysis of variance Kruskal–Wallis test). Hg, hemoglobin.
Figure 3.
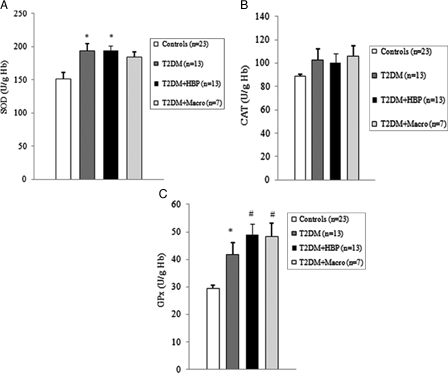
Antioxidant enzymatic activity is upregulated early in erythrocytes from type 2 diabetes patients. Superoxide dismutase (SOD) (A), catalase (CAT) (B), and glutathione peroxidase (GPX) (C) activity was measured in erythrocytes from type 2 diabetes patients at first clinical onset and from non-diabetes age-matched individuals. Values are mean ± SE. *P < 0.05, #P < 0.01 vs. controls (analysis of variance, one-way Tukey test).
Figure 4.
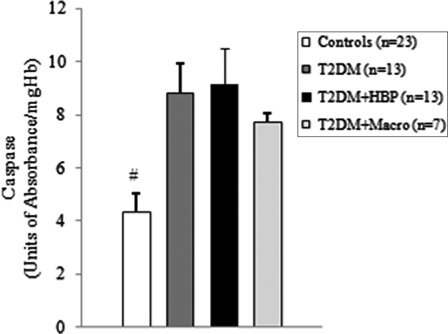
Caspase-3 is activated in RBCs of type 2 diabetes patients from first clinical onset. Caspase-3 activity was measured in RBCs from early type 2 diabetes patients and from non-diabetes age-matched subjects. Values are mean ± SE. #P < 0.05 vs. controls (analysis of variance Kruskal–Wallis test).
Discussion
We were able to show that ROS overproduction is present in type 2 diabetes patients at first clinical onset, as demonstrated by increased levels of lipid peroxides in blood samples (Figs. 1B and 2B). Our data are in line with previous studies that documented significantly increased levels of various markers of ROS in type 2 diabetes subjects.9–31 Moreover, we found that the antioxidant status of plasma increases early in diabetes (Fig. 1A). Similar results were published for plasma of late type 2 diabetes patients, irrespective of chronic complications.7,32 It has also been reported that different antioxidants can be generated in diabetes, depending on which ROS are produced.33 Therefore, plasma antioxidant status can vary, depending on which antioxidant is predominantly detected by the method used.34 In experimental diabetes, such differences were observed and correlated with plasma levels of vitamin E.34 Taking into consideration the ability of ROS, that is, superoxide and hydrogen peroxide,25,26 to permeate the erythrocyte membrane, we further investigated the intracellular antioxidant activity of our subjects. Interestingly, total antioxidant capacity increased in the RBCs of early type 2 diabetes patients (Fig. 2A). Moreover, we observed an upregulation of intracellular antioxidant enzymes (Fig. 3). The significant increase in only SOD and GPX activities could be a consequence of a tissue-specific modulation of the antioxidant enzymes.35 We were therefore able to show that, from early diabetes, RBCs are exposed to ROS overproduction originating either from inside or outside the cell, and they exhibit both an increased non-enzymatic and enzymatic antioxidant defense. Early activation of the antioxidant defense could be an argument for the controversial effects of antioxidant supplements in patients with diabetes.36–38 The compensatory efficiency of the response remains to be elucidated, especially when the oxidative stress-mediated shortened life span of RBCs in diabetes is taken into account.10 Our data show that early caspase-3 activity increases in RBCs of patients with type 2 diabetes (Fig. 4). Interestingly, we found a significant negative correlation between caspase-3 activity and the values of plasma MDA–TBA adducts in type 2 diabetes patients with hypertension (Spearman r = −0.662, P = 0.0219). Moreover, caspase-3 activity positively correlates with the levels of TEAC in RBCs from diabetes patients, especially in the hypertensive subgroup (Spearman r = 0.726 vs. 0.8172, and P = 0.0049 vs. 0.0019, respectively). This result suggests that lipid peroxides exhibit a strong inhibitory effect on caspase activity. A possible explanation is that the caspases are a family of cysteine proteases containing a thiol group as the active site necessary for their activity.39 This thiol group renders them particularly susceptible to redox modification by S-nitrosylation or oxidation. Such modifications result in the inhibition of their catalytic activities.40 On the other hand, as previously described in late type 2 diabetes,10 the lack of correlation between caspase-3 levels and intra-erythrocyte markers of oxidative stress suggests that the extracellular oxidative damage could be a critical modulator of the life span of RBCs from early diabetes.
In conclusion, our study shows that the pattern of defense against free radical aggression is activated early in the blood stream of type 2 diabetes patients, and occurs even in RBCs with a shortened life span. By showing that the antioxidant defense is upregulated from first clinical onset in type 2 diabetes, we question the beneficial effects of antioxidant supplements for clinical practice. Nevertheless, the evaluation of antioxidant status has to be considered for clinical applicability, despite the presence of any specific chronic complication due to diabetes.
Acknowledgements
The control group of subjects was recruited courtesy of Ioana Veronica Grajdeanu, Senior MD, Senior Lecturer, Department of Internal Medicine, University of Medicine and Pharmacy, Bucharest, Romania.
References
- 1.Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, et al.. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol 2007;583(Pt 1):9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaneto H, Katakami N, Kawamori D, Miyatsuka T, Sakamoto K, Matsuoka TA, et al.. Involvement of oxidative stress in the pathogenesis of diabetes. Antioxid Redox Signal 2007;9(3):355–66. [DOI] [PubMed] [Google Scholar]
- 3.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care 1996;19(3):257–67. [DOI] [PubMed] [Google Scholar]
- 4.Ceriello A. Oxidative stress and glycemic regulation. Metabolism 2000;49(2 Suppl 1):27–9. [DOI] [PubMed] [Google Scholar]
- 5.Opara EC. Oxidative stress, micronutrients, diabetes mellitus and its complications. J R Soc Promot Health 2002;122(1):28–34. [DOI] [PubMed] [Google Scholar]
- 6.Lodovici M, Bigagli E, Bardini G, Rotella CM. Lipoperoxidation and antioxidant capacity in patients with poorly controlled type 2 diabetes. Toxicol Ind Health 2009;25(4–5):337–41. [DOI] [PubMed] [Google Scholar]
- 7.El Boghdady NA, Badr GA. Evaluation of oxidative stress markers and vascular risk factors in patients with diabetic peripheral neuropathy. Cell Biochem Funct 2012;30(4):328–34. [DOI] [PubMed] [Google Scholar]
- 8.Sailaja YR, Baskar R, Saralakumari D. The antioxidant status during maturation of reticulocytes to erythrocytes in type 2 diabetics. Free Radic Biol Med 2003;35(2):133–9. [DOI] [PubMed] [Google Scholar]
- 9.Calderon-Salinas JV, Munoz-Reyes EG, Guerrero-Romero JF, Rodriguez-Moran M, Bracho-Riquelme RL, Carrera-Gracia MA, et al.. Eryptosis and oxidative damage in type 2 diabetic mellitus patients with chronic kidney disease. Mol Cell Biochem 2011;357(1–2):171–9. [DOI] [PubMed] [Google Scholar]
- 10.Maellaro E, Leoncini S, Moretti D, Del Bello B, Tanganelli I, De Felice C, et al.. Erythrocyte caspase-3 activation and oxidative imbalance in erythrocytes and plasma of type 2 diabetic patients. Acta Diabetol 2011;(1):1–7. [DOI] [PubMed] [Google Scholar]
- 11.King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol 2008;79(Suppl 8):1527–34. [DOI] [PubMed] [Google Scholar]
- 12.Kesavulu MM, Giri R, Kameswara Rao B, Apparao C. Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes Metab 2000;26(5):387–92. [PubMed] [Google Scholar]
- 13.Peuchant E, Delmas-Beauvieux MC, Couchouron A, Dubourg L, Thomas MJ, Perromat A, et al.. Short-term insulin therapy and normoglycemia. Effects on erythrocyte lipid peroxidation in NIDDM patients. Diabetes Care 1997;20(2):202–7. [DOI] [PubMed] [Google Scholar]
- 14.Sundaram RK, Bhaskar A, Vijayalingam S, Viswanathan M, Mohan R, Shanmugasundaram KR. Antioxidant status and lipid peroxidation in type II diabetes mellitus with and without complications. Clin Sci (Lond) 1996;90(4):255–60. [DOI] [PubMed] [Google Scholar]
- 15.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diagnosis and classification of diabetes mellitus. Diabetes Care 2004;27(Suppl 1):S5–10.14693921 [Google Scholar]
- 16.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 1990;186:407–21. [DOI] [PubMed] [Google Scholar]
- 17.Miller NJ, Johnston JD, Collis CS, Rice-Evans C. Serum total antioxidant activity after myocardial infarction. Ann Clin Biochem 1997;34(Pt 1):85–90. [DOI] [PubMed] [Google Scholar]
- 18.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26(9–10):1231–7. [DOI] [PubMed] [Google Scholar]
- 19.Ursini F, Maiorino M, Brigelius-Flohe R, Aumann KD, Roveri A, Schomburg D, et al.. Diversity of glutathione peroxidases. Methods Enzymol 1995;252:38–53. [DOI] [PubMed] [Google Scholar]
- 20.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974;47(3):469–74. [DOI] [PubMed] [Google Scholar]
- 21.Aebi H. Catalase in vitro. Methods Enzymol 1984;105:121–6. [DOI] [PubMed] [Google Scholar]
- 22.Kedziora-Kornatowska KZ, Luciak M, Blaszczyk J, Pawlak W. Lipid peroxidation and activities of antioxidant enzymes in erythrocytes of patients with non-insulin dependent diabetes with or without diabetic nephropathy. Nephrol Dial Transplant 1998;13(11):2829–32. [DOI] [PubMed] [Google Scholar]
- 23.Valkonen M, Kuusi T. Spectrophotometric assay for total peroxyl radical-trapping antioxidant potential in human serum. J Lipid Res 1997;38(4):823–33. [PubMed] [Google Scholar]
- 24.Denicola A, Souza JM, Radi R. Diffusion of peroxynitrite across erythrocyte membranes. Proc Natl Acad Sci U S A 1998;95(7):3566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch RE, Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem 1978;253(13):4697–9. [PubMed] [Google Scholar]
- 26.Mathai JC, Sitaramam V. Stretch sensitivity of transmembrane mobility of hydrogen peroxide through voids in the bilayer. Role of cardiolipin. J Biol Chem 1994;269(27):17784–93. [PubMed] [Google Scholar]
- 27.Ahmed FN, Naqvi FN, Shafiq F. Lipid peroxidation and serum antioxidant enzymes in patients with type 2 diabetes mellitus. Ann N Y Acad Sci 2006;1084:481–9. [DOI] [PubMed] [Google Scholar]
- 28.Prior RL, Cao G. In vivo total antioxidant capacity: comparison of different analytical methods. Free Radic Biol Med 1999;27(11–12):1173–81. [DOI] [PubMed] [Google Scholar]
- 29.Pasaoglu H, Sancak B, Bukan N. Lipid peroxidation and resistance to oxidation in patients with type 2 diabetes mellitus. Tohoku J Exp Med 2004;203(3):211–8. [DOI] [PubMed] [Google Scholar]
- 30.Aguirre F, Martin I, Grinspon D, Ruiz M, Hager A, De Paoli T, et al.. Oxidative damage, plasma antioxidant capacity, and glycemic control in elderly NIDDM patients. Free Radic Biol Med 1998;24(4):580–5. [DOI] [PubMed] [Google Scholar]
- 31.Nourooz-Zadeh J, Tajaddini-Sarmadi J, McCarthy S, Betteridge DJ, Wolff SP. Elevated levels of authentic plasma hydroperoxides in NIDDM. Diabetes 1995;44(9):1054–8. [DOI] [PubMed] [Google Scholar]
- 32.Guldiken B, Demir M, Guldiken S, Turgut N, Turgut B, Tugrul A. Oxidative stress and total antioxidant capacity in diabetic and nondiabetic acute ischemic stroke patients. Clin Appl Thromb Hemost 2009;15(6):695–700. [DOI] [PubMed] [Google Scholar]
- 33.Halliwell B, Gutteridge JM. The definition and measurement of antioxidants in biological systems. Free Radic Biol Med 1995;18(1):125–6. [DOI] [PubMed] [Google Scholar]
- 34.Feillet-Coudray C, Rock E, Coudray C, Grzelkowska K, Azais-Braesco V, Dardevet D, et al.. Lipid peroxidation and antioxidant status in experimental diabetes. Clin Chim Acta 1999;284(1):31–43. [DOI] [PubMed] [Google Scholar]
- 35.Wassmann S, Wassmann K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension 2004;44(4):381–6. [DOI] [PubMed] [Google Scholar]
- 36.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev 2012;3:CD007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neri S, Calvagno S, Mauceri B, Misseri M, Tsami A, Vecchio C, et al.. Effects of antioxidants on postprandial oxidative stress and endothelial dysfunction in subjects with impaired glucose tolerance and type 2 diabetes. Eur J Nutr 2010;49(7):409–16. [DOI] [PubMed] [Google Scholar]
- 38.Golbidi S, Ebadi SA, Laher I. Antioxidants in the treatment of diabetes. Curr Diabetes Rev 2011;7(2):106–25. [DOI] [PubMed] [Google Scholar]
- 39.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 1998;281(5381):1312–6. [DOI] [PubMed] [Google Scholar]
- 40.Curtin JF, Donovan M, Cotter TG. Regulation and measurement of oxidative stress in apoptosis. J Immunol Methods 2002;265(1–2):49–72. [DOI] [PubMed] [Google Scholar]


