Figure 2.
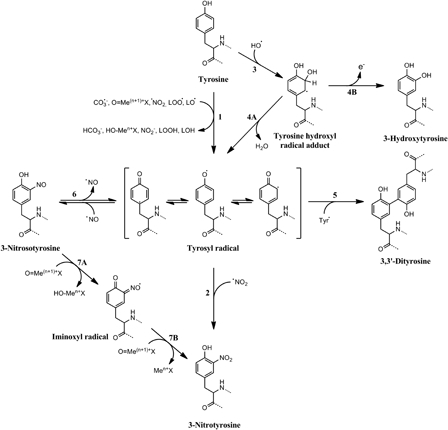
Free radical mechanisms of tyrosine nitration and oxidation. Several one-electron oxidants can oxidize tyrosine to tyrosyl radical (1), which can then react with nitrogen dioxide to produce 3-nitrotyrosine (2). Hydroxyl radical can add into the phenolic ring of tyrosine (3) yielding a tyrosine hydroxyl radical adduct that can dehydrate to tyrosyl radical (4A) or loose an electron to produce the stable product 3-hydroxytyrosine (4B). Tyrosyl radicals, besides reacting with •NO2, can recombine between themselves to generate the oxidation product 3,3′-dityrosine (5). An alternative route to tyrosine nitration implies the reaction of tyrosyl radicals with nitric oxide (6) to yield 3-nitrosotyrosine. This intermediate can be then oxidized to 3-nitrotyrosine by two steps of one-electron oxidation mediated by oxo-metal complexes. The first oxidation produces an iminoxyl radical intermediate (7A) that is finally oxidized to 3-nitrotyrosine (7B). Modified from Ref. 5.
