Figure 3.
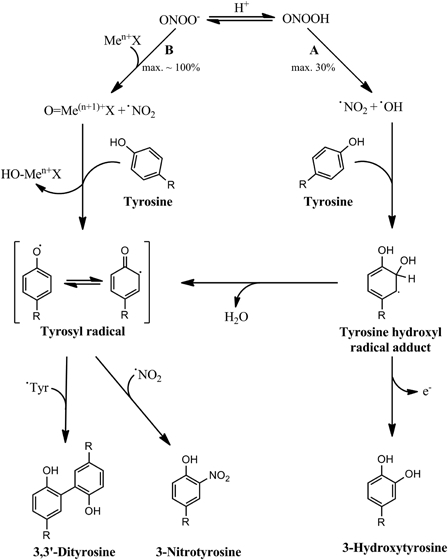
Peroxynitrite-dependent tyrosine nitration mediated by transition metal centers. In the absence of any direct target, peroxynitrous acid decays by homolysis producing nitrogen dioxide and hydroxyl radicals with maximum yields of 30% (A). These two radicals mediate tyrosine nitration and oxidation by a hydroxyl radical-dependent pathway that produces 3-nitrotyrosine in relatively low yields, as well as 3,3′-dityrosine and 3-hydroxytyrosine. However, in the presence of certain transition metal centers that react fast with ONOO−, almost all of the peroxynitrite present can decompose by reacting with the metal complex (B), producing high levels of the oxidized oxo-metal complex and nitrogen dioxide. In this case, these two species mediate tyrosine nitration, which can occur now in higher yields. Also, high levels of 3,3′-dityrosine may be produced.
