Abstract
Objectives: Salivary advanced glycation end-products (AGEs), advanced oxidation protein products (AOPP), total antioxidant capacity (TAC), and ferric reducing ability of saliva (FRAS) are increased in various diseases. Little data exist for these markers in the healthy population. The aim of this study was to assess the inter-individual and intra-individual variability of AGEs, AOPP, TAC, and FRAS in the saliva of young healthy individuals.
Methods: Unstimulated saliva samples were collected from 16 females and 18 males daily over a period of 30 days. Markers were measured using spectrophotometric and spectrofluorometric microplate-based methods.
Results: All salivary markers measured were significantly higher in men than in women (P < 0.05 for AGEs; P < 0.001 for AOPP, TAC, and FRAS). The inter-individual variability was approximately 60% for AGEs and AOPP and 30–40% for TAC and FRAS in both genders. The inter-individual variability of FRAS was higher in men vs. women (P < 0.01). Intra-individual variability ranged from 20% for TAC, to 30% for AGES and FRAS and 45% for AOPP.
Discussion: Intra-individual variability of salivary AGEs, AOPP, TAC, and FRAS indicates that their use is currently limited to large cohort studies. Identifying the underlying factors related to the high inter-individual and intra-individual variability is needed. Sex differences should be considered in future studies.
Keywords: Biomarkers of oxidative stress, Carbonyl stress, Sex difference
Introduction
Oxidative stress is defined as an imbalance of the pro-oxidant versus antioxidant balance in favor of the pro-oxidants.1 Its effects are considered not to be exclusively harmful, as a certain level of oxidative stress is indispensable for the normal function of various metabolic processes and signaling pathways.2 However, excessive and chronic oxidative stress can cause serious damage to lipids, nucleic acids, and proteins. Carbonyl stress is an irreversible form of non-enzymatic oxidation, when reducing sugars (or other carbonyl substances) react with amino groups of proteins and other molecules3 resulting in structural and functional changes.4
Oxidative and carbonyl stress are involved in the pathogenesis of several diseases and their complications.5 These include oral diseases such as caries,6 gingivitis,7 and periodontitis.8,9 Salivary markers of oxidative and carbonyl stress might thus be potentially useful in dentistry.
Advanced glycation end-products (AGEs), a marker of carbonyl stress, represent a heterogeneous group of compounds. Their sources include endogenous reactions, diet, and smoking.10,11 Auto-oxidation of glucose results in the formation of free radicals and hydrogen peroxide further potentiating the formation of AGEs.12 Binding of AGEs to specific receptors recognizing AGE-modified proteins (RAGE – receptor for advanced glycation end-products) leads to the activation of intracellular signaling cascades and an entire spectrum of proinflammatory and profibrotic cellular responses.13
Advanced oxidation protein products (AOPP) were identified as a marker of protein oxidation in 1996 in uremic patients.14 They are carried by oxidized proteins in plasma (especially albumin) and do not have oxidant properties themselves.14 On the contrary, fibrinogen that exerts antioxidant properties is one of the proteins reacting in AOPP reaction.15 The abundant content of dityrosines allows crosslinking through disulfide bridges and carbonyl groups.16 AOPP are formed mainly by chlorinated oxidants, i.e. hypochlorous acid and chloramines, as a result of myeloperoxidase activity of neutrophils,16 thus providing a marker of their inflammatory response.
The concentration of individual antioxidants (e.g. free –SH groups of proteins, uric acid, vitamins C and E, and bilirubin) can be measured in various biological fluids separately, but this is labor-intensive, costly, and time-consuming. An alternative approach is to measure the total antioxidant capacity (TAC). TAC is contributed to mostly by the total proteins (their –SH groups) and uric acid, in addition to the total bilirubin and vitamins C and E.17 Ferric reducing ability of saliva (FRAS) was modified from the FRAP (ferric reducing ability of plasma) assay, developed in 199618 to measure the ‘antioxidant power’ of human plasma. In addition to uric acid, the FRAP assay estimates other non-enzymatic antioxidants (e.g. vitamins C and E and bilirubin),19 but it cannot detect antioxidants containing –SH/thiol groups such as proteins and reduced glutathione.20
There is no ‘universal’ marker of oxidative stress as there are many causative oxidative agents and various molecules that are prone to oxidative damage. Due to the complex nature of oxidative stress, a wide palette of biomarkers has to be evaluated. This enables the understanding of the role of oxidative stress in disease pathogenesis and may be potentially used for disease screening or monitoring.21 In humans, saliva is easier to obtain than blood, especially in older people and children. Therefore, it is an attractive alternative diagnostic fluid.
Our group has assessed the variability of thiobarbituric acid-reacting substances in saliva in the past.22 The aim of this study was to analyze the intra-individual and inter-individual day-to-day variability of salivary AGEs, AOPP, TAC, and FRAS in young healthy volunteers. To our knowledge, this is the first study on the variability of a set of markers of oxidative and carbonyl stress in saliva.
Material and methods
Thirty-four young healthy volunteers (16 females, mean age 23.4 ± 3.0 years and 18 males, mean age 25.4 ± 3.1 years) were recruited for this study. The volunteers were instructed to collect whole unstimulated saliva into 2 ml tubes in the morning daily during a period of 30 consecutive days. Sampling was performed 30 minutes after tooth-brushing. Volunteers were instructed not to eat before the sampling. After collection, samples were frozen immediately and stored at –20°C until further measurements.
The study was approved by the Ethics Committee of the Institute of Molecular Biomedicine, Faculty of Medicine, Comenius University in Bratislava, Slovakia. All participants signed an informed consent form and filled in questionnaires to assess their health status (including acute or chronic illness) and history was taken to exclude smoking, alcohol consumption, the taking of medication, and artificial antioxidant supplements and sports activities that might influence the levels of oxidative stress markers.
Prior to the analysis of biochemical parameters, the samples were centrifuged (3000×g, 10 minutes at 4°C) and aliquots were transferred into 96-well microtitration plates. The calibration curves for each assay method and plate were prepared in triplicate. Intra-assay and inter-assay coefficients of variability (CV) of the methods were assessed as a measure of the respective assay reliability. All chemicals and reagents were purchased from Sigma-Aldrich (Germany) and the measurements were carried out on a Saphire II spectrofluorometer (Tecan, Austria).
Determination of AGEs was conducted using spectrofluorometry.23 Using black flat-bottom 96-well microtitration plates, 20 µl of samples followed by 180 µl of phosphate buffer saline (PBS, pH 7.4) were pipetted into corresponding wells. After short vortexing, the specific fluorescence (lambdaex.370 nm and lambdaem.440 nm) was measured using an AGE-BSA calibration system made by the rapid (4-days) incubation of bovine serum albumin (BSA; 50 mg/ml) with 0.5 mol/l glucose in 0.2 mol/l sodium phosphate buffer (pH 7.4) at 50°C using thermal glycation. AGEs concentration in the samples was expressed in g/l of saliva.
Measurement of AOPP was performed as described previously.24 Two hundred microliters of saliva and chloramine-T standards (0–100 µmol/l) were pipetted into a 96-well plate. Ten microliters of 1.16 mol/l KI were added only to the wells containing the standards and the plate was vortexed (6 minutes). Twenty microliters of glacial acetic acid was added and the plate was vortexed again (2 minutes). Absorbance at 340 nm was read immediately. The concentration of AOPP in the samples was expressed as μmol/l of chloramine-T equivalents.
TAC was analyzed according to a previously published protocol.23 Twenty microliters of samples or standards were mixed with 200 µl of reagent 1 (0.4 mol/l acetate buffer solution, pH 5.8). Blank absorbance at 660 nm was measured. Then, 20 µl of reagent 2 (30 mmol/l acetate buffer solution, pH 3.6) was added to each well. After 5 minutes, the plate was measured again at the same absorbance wavelength. The absorbances before adding reagent 2 were subtracted from those measured after its addition and the TAC concentration was expressed as μmol trolox equivalents/l (eq./l). The assay was calibrated with trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, 0–1000 µmol/l), a water-soluble derivative of vitamin E.
FRAS, a marker of non-protein antioxidant capacity, was measured according to a previously published protocol.6 Samples were incubated with TPTZ (2,4,6-tri(2-pyridyl)-s-triazine) in hydrochloric acid, ferric chloride, and acetate buffer. Reagents were prepared as follows: 3 mol/l acetate buffer (pH 3):10 mmol/l TPTZ in 40 mmol/l HCl:20 mmol/l FeCl3.6H2O:distilled water (1:1:1:9) to obtain the FRAS assay working reagent. The freshly prepared FRAS reagent (200 µl), warmed up to 37°C, was pipetted into each well of a 96-well plate and initial absorbance at 593 nm was read. Then 20 µl of samples or standards were added and the plate was vortexed briefly. After 4 minutes, the absorbance was read at 593 nm again. The initial absorbance values were subtracted from the absorbance after adding samples and standards. Absorbance readings at 593 nm were plotted against a calibration curve of ferrous sulfate (FeSO4.7H2O, 0–1000 µmol/l). FRAS values of saliva samples were expressed in µmol/l saliva.
Statistical analyses
Statistical analyses were performed using GraphPad Prism (version 6.01, USA) and IBM SPSS Statistics 21 software (USA). Data were analyzed with the Mann–Whitney U test, one-way ANOVA, repeated measures two-way ANOVA (with Bonferroni correction), and the F-test of equality of variances for the comparison of between-gender variability. Outliers were detected using the Grubbs’ test and removed from further analyses. A value of P < 0.05 was considered statistically significant.
Results
Mean values, with their corresponding standard deviations for all parameters, along with the coefficients of variation for inter-individual variability and the range of intra-individual variability are summarized in Table 1.
Table 1. Descriptive statistics of all measured parameters.
| Mean and SD | CV (inter-individual) (%) | CV (intra-individual) – Range (%) | ||||
|---|---|---|---|---|---|---|
| Parameter | Females | Males | Females | Males | Females | Males |
| AGEs (g/l) | 0.23 ± 0.13 | 0.27 ± 0.19* | 56.08 | 69.15 | 17.45–69.22 | 21.21–62.53 |
| AOPP (μmol/l) | 29.27 ± 17.77 | 37.68 ± 23.33** | 60.72 | 61.92 | 32.56–61.02 | 29.61–122.75 |
| TAC (μmol trolox eq./l) | 531.3 ± 149.9 | 582.2 ± 173.7** | 28.21 | 29.83 | 8.00–38.11 | 3.08–57.28 |
| FRAS (μmol/l) | 321.3 ± 138.1 | 459.7 ± 221.4** | 42.96 | 48.15 | 16.24–84.78 | 13.06–113.59 |
Mean and standard deviation, inter-individual and intra-individual variability. AGEs – advanced glycation end-products, AOPP – advanced oxidation protein products, TAC – total antioxidant capacity, FRAS – ferric reducing ability of saliva, SD – standard deviation, and CV – coefficient of variation.
*P < 0.05.
**P < 0.001 when compared to females.
Mean salivary levels of AGEs, AOPP, TAC, and FRAS varied significantly between males and females (Fig. 1A; P < 0.05 for AGEs and Fig. 1B–D; P < 0.001 for AOPP, TAC, and FRAS, respectively). Repeated measures two-way ANOVA showed the significant influence of sampling day as a factor affecting the variability of salivary AOPP (Table 2; F = 1.764; P < 0.01) but not AGEs, TAC, or FRAS. Gender as a factor affecting variability was found to be statistically significant only in salivary FRAS (Table 2; F = 6.743; P < 0.05).
Figure 1.
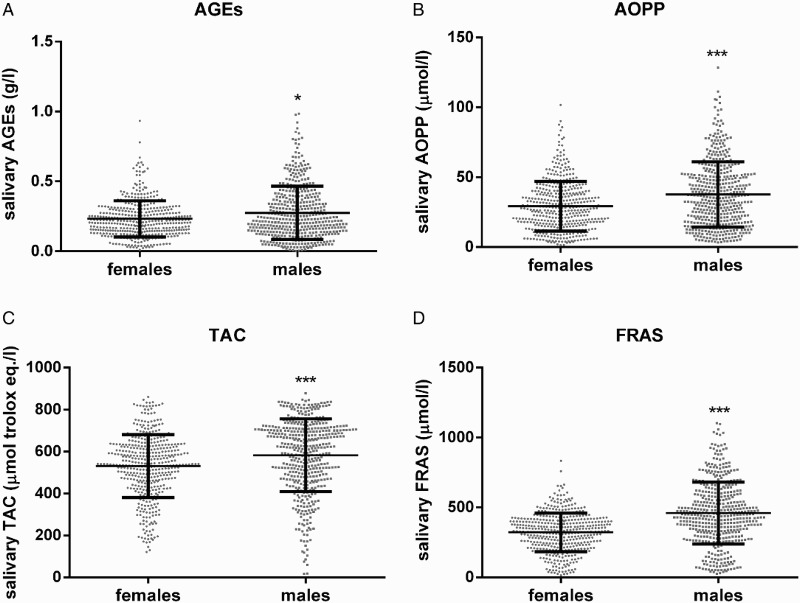
Salivary AGEs (A), AOPP (B), TAC (C), and FRAS (D) levels in females and males. All analyzed samples are plotted. AGEs – advanced glycation end-products, AOPP – advanced oxidation protein products, TAC – total antioxidant capacity, and FRAS – ferric reducing ability of saliva. * denotes P < 0.05, *** denotes P < 0.001 when compared to females.
Table 2. Repeated measures two-way ANOVA – selected components of variability.
| Gender | Day of sampling | |
|---|---|---|
| AGEs | F = 0.1495 | F = 0.9061 |
| P = 0.7019 | P = 0.6101 | |
| ns | ns | |
| AOPP | F = 2.415 | F = 1.764 |
| P = 0.1310 | P = 0.0081 | |
| ns | ** | |
| TAC | F = 1.031 | F = 1.187 |
| P = 0.3182 | P = 0.2291 | |
| ns | ns | |
| FRAS | F = 6.743 | F = 0.8548 |
| P = 0.0146 | P = 0.6876 | |
| * | ns |
AGEs – advanced glycation end-products, AOPP – advanced oxidation protein products, TAC – total antioxidant capacity, FRAS – ferric reducing ability of saliva, F – F-ratio, P – P value, and ns – not significant.
*P < 0.05.
**P < 0.01.
The intra-individual variability had a wide range for both genders (Table 1) and no significant gender difference was found. Coefficients of variations of more than 20% for TAC, more than 30% for AGEs and FRAS and more than 45% for AOPP were observed (Fig. 2).
Figure 2.
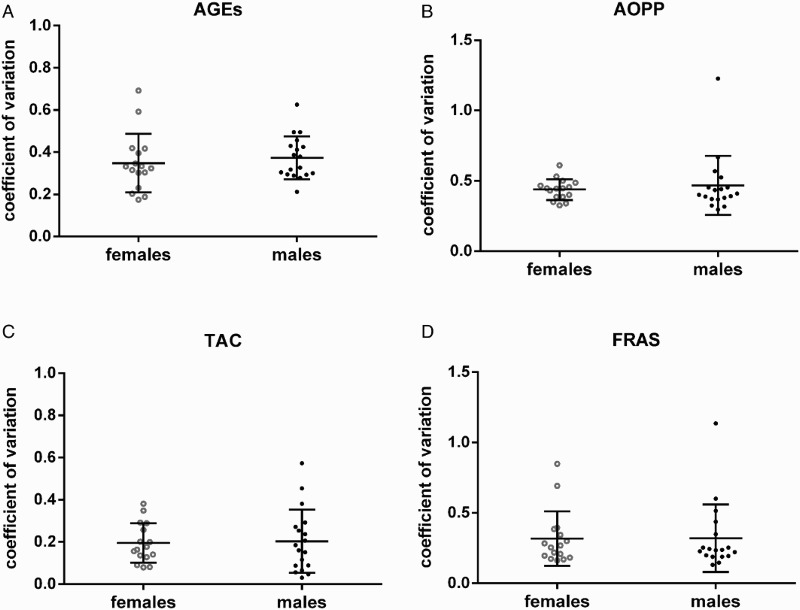
Intra-individual variability of AGEs (A), AOPP (B), TAC (C), and FRAS (D). AGEs – advanced glycation end-products, AOPP – advanced oxidation protein products, TAC – total antioxidant capacity, and FRAS – ferric reducing ability of saliva.
Concentrations of all measured markers varied considerably between the subjects (AGEs: F = 50.93, P < 0.0001; AOPP: F = 24.72, P < 0.0001; TAC: F = 40.22, P < 0.0001; and FRAS: F = 54.19, P < 0.0001). Inter-individual variability of AGEs and AOPP was very high in both genders, reaching 56.08% for females and 69.15% for males for AGEs, and 60.72% for females and 61.92% for males for AOPP. For TAC, the intra-individual variability was 28.21 and 29.83% (females vs. males, respectively) and 42.96 and 48.15% for FRAS (females vs. males, respectively). Additionally, the inter-individual variability of FRAS was higher in males when compared to females (Table 1; 48 vs. 43%; F = 4.956; P < 0.01).
The calculated intra-assay and inter-assay coefficients of variation were, for AGEs: intra-assay CV 3.47%, inter-assay CV 7.00%; AOPP: intra-assay CV 4.38%, inter-assay CV 8.21%; TAC: intra-assay CV 5.39%, inter-assay CV 6.78%; FRAS: intra-assay CV 5.20%, inter-assay CV 7.58%. These inter- and intra-assay CV values are sufficiently low to use these microplate methods for high-throughput measurement of AGEs, AOPP, TAC, and FRAS concentrations in saliva.
Discussion
In the present study, an extremely high intra-individual and inter-individual variability of salivary AGEs, AOPP, TAC, and FRAS was found, making the interpretation of individual values difficult. From previous studies, the biological variability in saliva is known to be higher than in plasma. There are only a few studies focusing on the comparison of oxidative stress markers in saliva and plasma. A positive plasma-saliva correlation was observed for FRAS in children.25 For TAC, no correlation between plasma and salivary levels was observed.26 For AOPP, a positive correlation between plasma and saliva was described as well.27 Several studies have shown that the oxidative stress markers are not transferred from plasma to saliva, and therefore, the salivary levels reflect the local oxidative stress in the salivary glands, rather than the systemic oxidative stress.28 Free radicals and reactive oxygen species in the oral cavity probably originate mainly from the polymorphonuclear neutrophils, which control growth of oral bacteria via mechanisms involving ROS.29 Under resting conditions, parotid saliva was identified to be the major source of salivary antioxidants.30 Uric acid contributes to the antioxidant capacity of saliva by approximately 70%.31 Therefore, it was proposed that saliva might represent a first line of defense against oxidative stress.
Concise data on oxidative stress markers in healthy subjects with regard to gender are scarce. Most of the published studies have been aimed at assessing these markers in blood or urine.32 These studies have focused on possible diurnal,33 day to day, and seasonal34 variations of oxidative stress in different biofluids or tissues, but not in saliva. In this study, lower TAC and FRAS values in females compared to males were observed. This observation is in agreement with others35 describing significantly lower salivary total antioxidant status in women than men, regardless of periodontal health. Gender differences in markers of oxidative stress and enzymatic antioxidant defense are usually attributed to differences in sex hormones, especially estradiol. It has been reported that women tend to have lower post-prandial levels of oxidative stress than men which might be explained by the antioxidative properties of estrogen.36 Also, higher antioxidant enzyme activities were reported in blood, liver, and brain of females of various species (compared with males), suggesting that oxidative damage and oxidative stress should be generally lower in females.37 However, there are studies reporting no effect of gender on oxidative stress in healthy subjects for saliva38 or blood.39 A possible explanation for the lower TAC and FRAS in women, observed in our and other studies, may be explained by lower unstimulated salivary flow in females due to smaller size of salivary glands based on their smaller body size.40
One of the limitations of the present study is its cross-sectional design. It is, thus, difficult to characterize the causes of increased oxidative stress and antioxidant status in males compared to females. Our study is purely observational, describing the variability of oxidative stress markers in young healthy individuals. General and local oral cavity health status and behavioral habits (including oral hygiene) were assessed only on the basis of the questionnaire. The effects of the seasonal and diurnal variations on our results can be excluded, since the sampling was done at the same time and same season in all subjects. Indeed, other multiple factors already identified such as tooth-brushing, daytime of sampling, smoking, and ascorbic acid treatment were shown to affect the levels of lipoperoxidation markers in saliva of healthy subjects.38 In our study, these were addressed in terms of same conditions; however, diet, composition of oral microbial flora, and genetic polymorphisms should be assessed in further studies. Our study did not deal with these factors.
Saliva is gaining importance in research and interest in the clinics as a diagnostic fluid, mainly because of its easy and non-invasive collection. As oxidative stress is involved in a number of local oral and systematic diseases, there are numerous clinical applications for the measurement of oxidative stress in saliva in terms of screening or disease activity monitoring. However, our results point to a major problem – the interpretation of the test results in an individual patient is complicated by the high biological variability. It can, thus, be currently purposed to use the whole palette of available oxidative stress markers in saliva or to study the factors that affect the inter-individual and intra-individual variability, so that they can be taken into account when collecting samples. The more challenging alternative is to search for novel markers of oxidative stress in saliva that would be more specific, less variable, and still easy to use for point-of-care testing.
In conclusion, the results presented in this paper are, to the best of our knowledge, the first attempt to characterize the variability of AGEs, AOPP, TAC, and FRAS in the saliva of young, healthy volunteers. Further studies are needed to explore the mechanism and the causes of the existing variability, as well as the exact origin of oxidative stress markers in saliva. Our results could be important for further research of diseases with regard to oxidative stress in saliva.
Disclaimer statements
Contributors IL measured the samples, analyzed the data and wrote the manuscript. LT measured the samples, analyzed the data and wrote the manuscript. JH designed the study, collected the samples and corrected the manuscript. MB collected the samples. PC designed the study, analyzed the data and critically reviewed the manuscript.
Funding This publication is the result of the implementation of the project University Science Park of Comenius University in Bratislava (ITMS 26240220086) supported by the Research and Development Operational Programme funded by the European Regional Development Fund.
Conflict of interest None.
Ethics approval The study was approved by the Ethics Committee of the Institute of Molecular Biomedicine, Faculty of Medicine, Comenius University in Bratislava, Slovakia.
References
- 1.Sies H. Oxidative stress II. Oxidants and antioxidants. London: Academic Press; 1991. [Google Scholar]
- 2.Lyu BN, Lyu MB, Ismailov BI, Ismailov SB. Four hypotheses on mitochondria's role in the development and regulation of oxidative stress in the normal state, cell pathology and reversion of tumor cells. Med Hypotheses 2007;69(1):186–94. doi: 10.1016/j.mehy.2006.10.055 [DOI] [PubMed] [Google Scholar]
- 3.Bargnoux AS, Morena M, Badiou S, Dupuy AM, Canaud B, Cristol JP. Carbonyl stress and oxidatively modified proteins in chronic renal failure [Article in French]. Ann Biol Clin (Paris) 2009;67:153–8. [DOI] [PubMed] [Google Scholar]
- 4.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 1997;272(33):20313–6. doi: 10.1074/jbc.272.33.20313 [DOI] [PubMed] [Google Scholar]
- 5.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 6.Tóthová L, Celecová V, Celec P. Salivary markers of oxidative stress and their relation to periodontal and dental status in children. Dis Markers 2013;34(1):9–15. doi: 10.1155/2013/591765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celec P, Cervenka T, Hodosy J, Boor P, Vesela S, Halcak L, et al. . Thiobarbituric acid reacting substances in saliva and their relation to the gingival inflammation. TMJ 2004;54(1):81–5. [Google Scholar]
- 8.D'Aiuto F, Nibali L, Parkar M, Patel K, Suvan J, Donos N. Oxidative stress, systemic inflammation, and severe periodontitis. J Dent Res 2010;89(11):1241–6. doi: 10.1177/0022034510375830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei D, Zhang XL, Wang YZ, Yang CX, Chen G. Lipid peroxidation levels, total oxidant status and superoxide dismutase in serum, saliva and gingival crevicular fluid in chronic periodontitis patients before and after periodontal therapy. Aust Dent J 2010;55(1):70–8. doi: 10.1111/j.1834-7819.2009.01123.x [DOI] [PubMed] [Google Scholar]
- 10.Uribarri J, Cai W, Sandu O, Peppa M, Goldberg T, Vlassara H. Diet-derived advanced glycation end products are major contributors to the body's AGE pool and induce inflammation in healthy subjects. Ann NY Acad Sci 2005;1043:461–6. doi: 10.1196/annals.1333.052 [DOI] [PubMed] [Google Scholar]
- 11.Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, et al. . Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci USA 1997;94(25):13915–20. doi: 10.1073/pnas.94.25.13915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff SP, Jiang ZY, Hunt JV. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med 1991;10:339–52. doi: 10.1016/0891-5849(91)90040-A [DOI] [PubMed] [Google Scholar]
- 13.Miyata T, Kurokawa K, van Ypersele de Strihou C. Advanced glycation and lipoxidation end products: role of reactive carbonyl compounds generated during carbohydrate and lipid metabolism. J Am Soc Nephrol 2000;11:1744–52. [DOI] [PubMed] [Google Scholar]
- 14.Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. . Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 1996;49:1304–13. doi: 10.1038/ki.1996.186 [DOI] [PubMed] [Google Scholar]
- 15.Selmeci L. Advanced oxidation protein products (AOPP): novel uremic toxins, or components of the non-enzymatic antioxidant system of the plasma proteome? Free Radic Res 2011;45(10):1115–23. doi: 10.3109/10715762.2011.602074 [DOI] [PubMed] [Google Scholar]
- 16.Capeillère-Blandin C, Gausson V, Descamps-Latscha B, Witko-Sarsat V. Biochemical and spectrophotometric significance of advanced oxidized protein products. Biochim Biophys Acta 2004;1689(2):91–102. doi: 10.1016/j.bbadis.2004.02.008 [DOI] [PubMed] [Google Scholar]
- 17.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 2004;37:277–85. doi: 10.1016/j.clinbiochem.2003.11.015 [DOI] [PubMed] [Google Scholar]
- 18.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem 1996;15:70–6. doi: 10.1006/abio.1996.0292 [DOI] [PubMed] [Google Scholar]
- 19.Ergun S, Troşala SC, Warnakulasuriya S, Özel S, Önal AE, Ofluoğlu D, et al. . Evaluation of oxidative stress and antioxidant profile in patients with oral lichen planus. J Oral Pathol Med 2011;40(4):286–93. doi: 10.1111/j.1600-0714.2010.00955.x [DOI] [PubMed] [Google Scholar]
- 20.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem 2005;53(6):1841–56. doi: 10.1021/jf030723c [DOI] [PubMed] [Google Scholar]
- 21.Čolak E. New markers of oxidative damage to macromolecules (review article). JMB 2008;27:1–16. [Google Scholar]
- 22.Behuliak M, Pálffy R, Gardlík R, Hodosy J, Halčák L, Celec P. Variability of thiobarbituric acid reacting substances in saliva. Dis Markers 2009;26:49–53. doi: 10.1155/2009/175683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celecová V, Kamodyová N, Tóthová L, Kúdela M, Celec P. Salivary markers of oxidative stress are related to age and oral health in adult non-smokers. J Oral Pathol Med 2013;42(3):263–6. doi: 10.1111/jop.12008 [DOI] [PubMed] [Google Scholar]
- 24.Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Capeillère-Blandin C, Nguyen AT, Canteloup S, et al. . Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol 1998;161(5):2524–32. [PubMed] [Google Scholar]
- 25.Lyszczarz R, Stypułkowska J, Stepniewski M, Szot WM. [Evaluation of saliva antioxidant activity for determining the state of dentition and oral hygiene in a group of young athletes]. [Article in Polish]. Wiad Lek 2002;55(Suppl 1(Pt 2)):768–72. [PubMed] [Google Scholar]
- 26.Youssef H, Groussard C, Machefer G, Minella O, Couillard A, Knight J, et al. . Comparison of total antioxidant capacity of salivary, capillary and venous samplings: interest of the salivary total antioxidant capacity on triathletes during training season. J Sports Med Phys Fitness 2008;48(4):522–9. [PubMed] [Google Scholar]
- 27.Qing Z, Ling-Ling E, Dong-Sheng W, Hong-Chen L. Relationship of advanced oxidative protein products in human saliva and plasma: age- and gender-related changes and stability during storage. Free Radic Res 2012;46(10):1201–6. doi: 10.3109/10715762.2012.700113 [DOI] [PubMed] [Google Scholar]
- 28.Iannitti T, Rottigni V, Palmieri B. Role of free radicals and antioxidant defences in oral cavity-related pathologies. J Oral Pathol Med 2012;41(9):649–61. doi: 10.1111/j.1600-0714.2012.01143.x [DOI] [PubMed] [Google Scholar]
- 29.Battino M, Ferreiro MS, Gallardo I, Newman HN, Bullon P. The antioxidant capacity of saliva. J Clin Periodontol 2002;29(3):189–94. doi: 10.1034/j.1600-051X.2002.290301x.x [DOI] [PubMed] [Google Scholar]
- 30.Nagler RM, Klein I, Zarzhevsky N, Drigues N, Reznick AZ. Characterization of the differentiated antioxidant profile of human saliva. Free Radic Biol Med 2002;32(3):268–77. doi: 10.1016/S0891-5849(01)00806-1 [DOI] [PubMed] [Google Scholar]
- 31.Moore S, Calder KA, Miller NJ, Rice-Evans CA. Antioxidant activity of saliva and periodontal disease. Free Radic Res 1994;21(6):417–25. doi: 10.3109/10715769409056594 [DOI] [PubMed] [Google Scholar]
- 32.Block G, Dietrich M, Norkus E, Jensen C, Benowitz NL, Morrow JD, et al. . Intraindividual variability of plasma antioxidants, markers of oxidative stress, C-reactive protein, cotinine, and other biomarkers. Epidemiology 2006;17(4):404–12. doi: 10.1097/01.ede.0000220655.53323.e9 [DOI] [PubMed] [Google Scholar]
- 33.Kanabrocki EL, Murray D, Hermida RC, Scott GS, Bremner WF, Ryan MD, et al. . Circadian variation in oxidative stress markers in healthy and type II diabetic men. Chronobiol Int 2002;19(2):423–39. doi: 10.1081/CBI-120002914 [DOI] [PubMed] [Google Scholar]
- 34.Balog T, Sobocanec S, Sverko V, Krolo I, Rocić B, Marotti M, et al. . The influence of season on oxidant–antioxidant status in trained and sedentary subjects. Life Sci 2006;78(13):1441–7. doi: 10.1016/j.lfs.2005.07.039 [DOI] [PubMed] [Google Scholar]
- 35.Sculley DV, Langley-Evans SC. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin Sci (Lond) 2003;105(2):167–72. doi: 10.1042/CS20030031 [DOI] [PubMed] [Google Scholar]
- 36.Bloomer RJ, Fisher-Wellman KH. Lower postprandial oxidative stress in women compared with men. Gend Med 2010;7(4):340–9. doi: 10.1016/j.genm.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 37.Bloomer RJ, Fisher-Wellman KH. Blood oxidative stress biomarkers: influence of sex, exercise training status, and dietary intake. Gend Med 2008;5(3):218–28. doi: 10.1016/j.genm.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 38.Kamodyová N, Tóthová L, Celec P. Salivary markers of oxidative stress and antioxidant status: influence of external factors. Dis Markers 2013;34(5):313–21. doi: 10.1155/2013/341302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pepe H, Balci SS, Revan S, Akalin PP, Kurtoğlu F. Comparison of oxidative stress and antioxidant capacity before and after running exercises in both sexes. Gend Med 2009;6(4):587–95. doi: 10.1016/j.genm.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 40.Inoue H, Ono K, Masuda W, Morimoto Y, Tanaka T, Yokota M, et al. . Gender difference in unstimulated whole saliva flow rate and salivary gland sizes. Arch Oral Biol 2006;51(12):1055–60. doi: 10.1016/j.archoralbio.2006.06.010 [DOI] [PubMed] [Google Scholar]


