Abstract
Objectives: 3-Methyl-1-phenyl-2-pyrazolin-5-one (edaravone) is used in clinical treatment of acute brain infarction to rescue the penumbra, based on its ability to prevent lipid peroxidation by scavenging lipid peroxyl radicals. Here, we show that edaravone also reacts with peroxynitrite to yield 4-NO-edaravone as the major product and 4-NO2-edaravone as a minor product.
Results: We observed little formation of 3-methyl-1-phenyl-2-pyrazolin-4,5-dione (4-oxoedaravone) and its hydrate, 2-oxo-3-(phenylhydrazono)butanoic acid, which are the major free radical-induced oxidation products of edaravone, suggesting that free radicals are not involved in the reaction with peroxynitrite. The reaction of peroxynitrite with edaravone is approximately 30-fold greater than with uric acid, a physiological peroxynitrite scavenger (reaction rate k = 1.5 × 104 M−1 s−1 vs. 480 M−1 s−1).
Discussion: These results suggest that edaravone functions therapeutically as a scavenger of peroxynitrite as well as lipid peroxyl radicals, which is consistent with a report that edaravone treatment reduced levels of 3-nitrotyrosine in the cerebrospinal fluid of patients with amyotrophic lateral sclerosis.
Keywords: Edaravone, Peroxynitrite, 4-NO-edaravone, 4-NO2-edaravone, Uric acid
Introduction
Oxygen radicals play important roles in causing ischemic and post-ischemic damage in the brain,1,2 and free radical scavengers are expected to be beneficial for rescuing the penumbra from injury. Indeed, intravenous injection of 3-methyl-1-phenyl-2-pyrazolin-5-one (edaravone: Fig. 1)3 at a dose of 30 mg twice a day for 14 days in patients with acute ischemic stroke, commencing within 24 hours after onset, significantly improved the functional outcome in a multicenter, randomized, placebo-controlled, double-blind study.4 Accordingly, in 2001 the Japanese Ministry of Health, Labor, and Welfare approved intravenous infusion of 30 mg edaravone twice a day for a maximum of 14 days for patients with acute brain infarction within 24 hours after onset, and edaravone is now widely used in Japan in the treatment of acute stroke.
Figure 1.
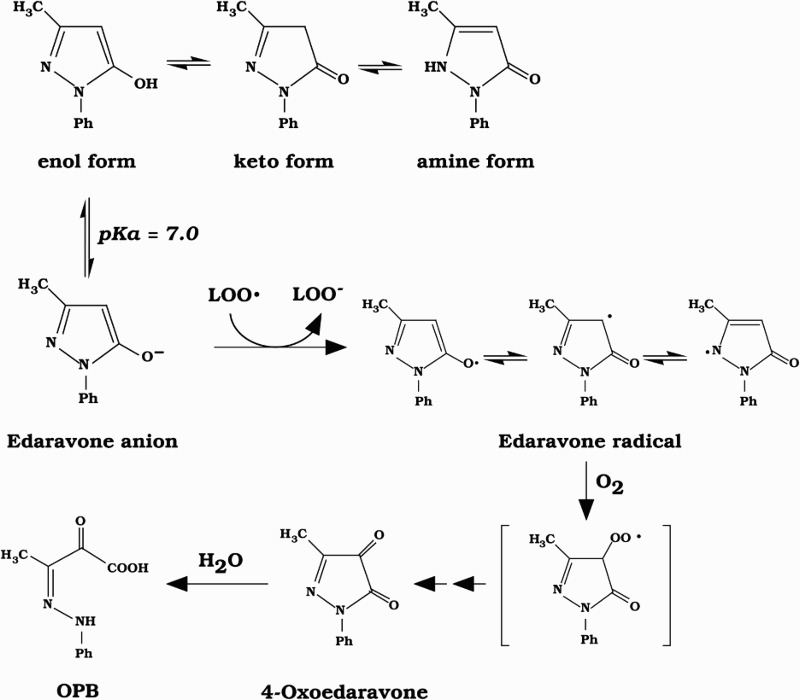
Pathways for the reduction of lipid peroxyl radicals (LOO•) by the edaravone anion and formation of its oxidation products.
Edaravone inhibits lipid peroxidation as efficiently as do well-known antioxidants such as vitamin E (VE) and ascorbic acid (VC).3 Furthermore, a combination of edaravone with VC or VE almost completely inhibited the oxidation of phosphatidylcholine liposomal membrane initiated with lipid-soluble and water-soluble azo initiators.3 The pKa value of edaravone is 7.0, and the rate of edaravone oxidation initiated with an azo compound increases with increasing pH, suggesting that the anionic form of edaravone is more reactive than the non-dissociated form.3 Edaravone anion is converted to the edaravone radical, which is oxidized to produce 3-methyl-1-phenyl-2-pyrazolin-4,5-dione (4-oxoedaravone) and its hydrolysate, 2-oxo-3-(phenylhydrazono)butanoic acid (OPB), as major products of peroxyl radical-induced oxidation (Fig. 1).3,5
The neuroprotective effect of edaravone has been confirmed in various animal models.6–8 Evidence that edaravone effectively scavenges free radicals under post-stroke conditions was attained by quantifying the formation of 14C-labeled OPB when 14C-labeled edaravone was infused following occlusion of the rat middle cerebral artery (MCA) and subsequent reperfusion.9 We revealed that sustained free radical-induced oxidative damage is evident in the brain for at least 7 days after MCA occlusion and reperfusion, and repeated intravenous infusion of edaravone for 14 days significantly reduced ischemic damage to the brain.10
Peroxynitrite (ONOO−), a powerful oxidant and nitrating agent formed by the reaction of nitric oxide (•NO) and superoxide anion (), contributes significantly to ischemia/reperfusion injury.11–16 The rate constant for this reaction is reported to be 1.6 × 1010 M−1 s−1, indicating that the reaction is diffusion-controlled.17 Peroxynitrite reacts with tyrosine residues in proteins to yield 3-nitrotyrosine residues,18 such that 3-nitrotyrosine is a good marker of peroxynitrite formation in vivo. 3-Nitrotyrosine has been detected in patients with cardiac and vascular diseases, as well as diabetes and diabetic complications.18
Although edaravone is known to react with peroxynitrite,19,20 its chemical features have not as yet been reported. Here, we show that edaravone reacts with peroxynitrite much faster than does uric acid, a good physiological scavenger of peroxynitrite,21–23 to produce 4-NO-edaravone as the major reaction product.
Materials and methods
Materials
Edaravone, uric acid, hydrogen peroxide, and sodium nitrite were obtained from Wako Pure Chemical (Osaka, Japan). The peroxynitrite generator 3-(4-morpholinyl)sydnonimine hydrochloride (SIN-1) was purchased from Dojindo (Kumamoto, Japan). Diethylenetriaminepentaacetic acid (DETAPAC) was obtained from Tokyo Kasei Kogyo (Tokyo, Japan). 4-Oxoedaravone, OPB, 4-NO-edaravone, and 4-NO2-edaravone were kind gifts from Mitsubishi Tanabe Pharma Corporation (Osaka, Japan).
Identification of reaction products of edaravone with peroxynitrite by LC/MS
Edaravone (1.0 mM) was incubated with 1.0 mM SIN-1 in 20 mM phosphate buffer (pH 7.4) containing 50% methanol and 50 µM DETAPAC for 6 hours at 37°C, and then the reaction mixture was analyzed by LC/MS. A N(CH3)2 column (250 mm × 4.6 mm i.d., Senshu Scientific, Tokyo, Japan) was used for product separation with 80% methanol containing 5.0 mM ammonium acetate as the mobile phase, delivered at 1.0 ml/min. Twenty percent of the column eluate was introduced into a Waters ZQ2000 quadrupole mass spectrometer with negative electrospray ionization set at −2500 V. The cone potential was maintained at −30 V and the temperature of the N2 drying gas was 350°C. Quantified solutions of 4-NO-edaravone and 4-NO2-edaravone standards were analyzed under identical conditions.
In a parallel experiment, instead of SIN-1, hydrogen peroxide and sodium nitrite were used to generate peroxynitrite.24 Identical procedures were conducted as above.
The time courses of edaravone decay and formation of products were determined using the above conditions. 4-Oxoedaravone and OPB were analyzed as previously described.3
Competitive reaction of edaravone and uric acid with peroxynitrite
Edaravone and uric acid (50 µM each) were added to 50 µM SIN-1 in 20 mM phosphate buffer (pH 7.4) containing 50% methanol and 50 µM DETAPAC under aerobic conditions at 37°C. Edaravone concentration was quantified by reverse-phase HPLC analysis with electrochemical detection as summarized; the mobile phase was 50 mM NaClO4 in methanol/water (60:40, v/v), delivered at 0.8 ml/min; the separation column was a CAPCELL PAK C18 column (5 µm, 250 mm × 4.6 mm i.d., Shiseido, Japan). The applied voltage to the electrochemical detector was 700 mV against an Ag/AgCl reference electrode. Uric acid was measured on a LC-NH2 column (5 µm, 250 mm × 4.6 mm i.d.; Supelco, USA) using the same mobile phase and electrochemical detector conditions as detailed above.
Determination of the rate constant of edaravone reaction with peroxynitrite
To determine the rate constant (k E) of the reaction of edaravone with peroxynitrite, we performed a competitive reaction of edaravone and uric acid with peroxynitrite, since the rate constant (kUA) of uric acid with peroxynitrite is established to be 480 M−1 s−1.21 The rate constants were calculated as the ratio of each substrate concentration divided by initial concentrations, according to the equation:
Results and discussion
Reaction products of edaravone with peroxynitrite
Figure 2A shows the HPLC–UV (240 nm) chromatogram analysis of the reaction solution obtained when 1.0 mM edaravone was incubated with 1.0 mM SIN-1 in 20 mM phosphate buffer (pH 7.4) containing 50% methanol and 50 µM DETAPAC under aerobic conditions at 37 °C for 1 hour. Two products were detected and eluted at 7.9 and 13.8 minutes as major and minor products, respectively. For comparison, authentic 4-NO-edaravone and 4-NO2-edaravone were eluted also at 7.9 and 13.8 minutes, respectively (Fig. 2B and C). Additionally, the MS spectra of compounds eluted at 7.9 and 13.8 minutes were identical to those of 4-NO-edaravone and 4-NO2-edaravone, respectively (Fig. 3A and B). We contend that the major and minor products in the reaction of edaravone with peroxynitrite are 4-NO-edaravone and 4-NO2-edaravone, respectively. We confirmed that reaction of edaravone with peroxynitrite prepared from hydrogen peroxide and sodium nitrite gave identical products (data not shown).
Figure 2.
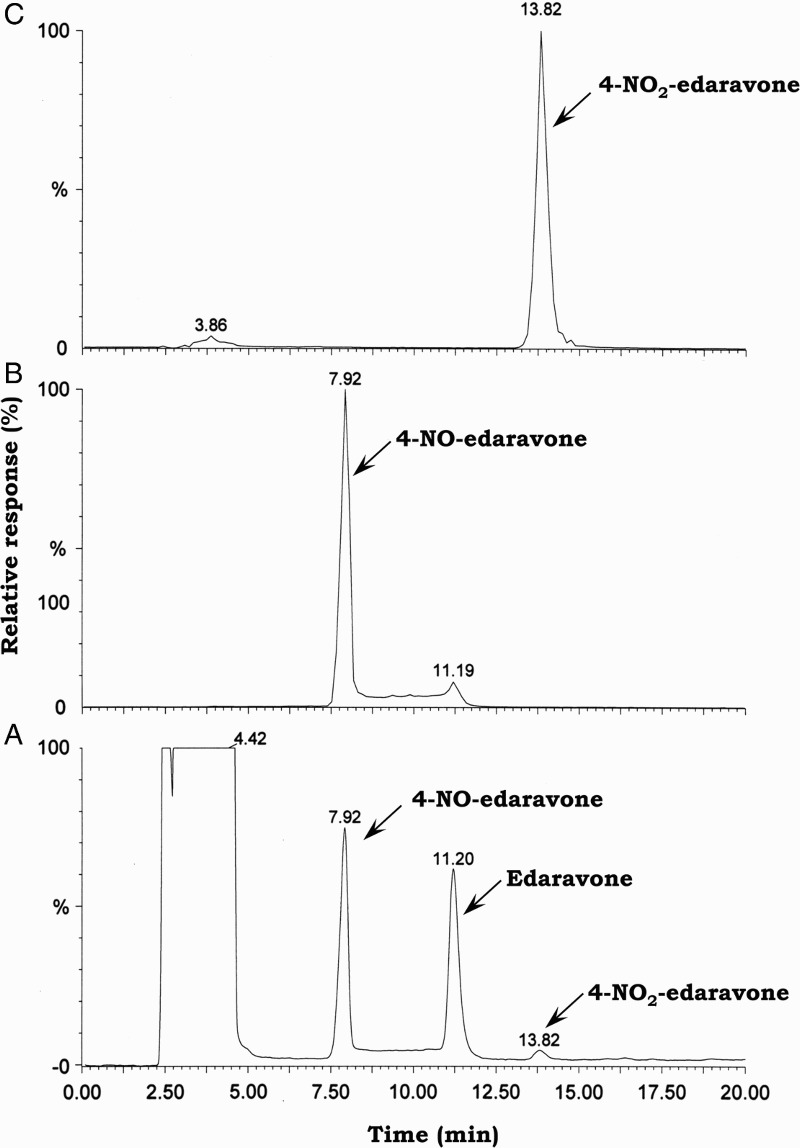
A HPLC–UV (240 nm) chromatogram (A) of the reaction solution obtained by treating 1.0 mM edaravone with 1.0 mM SIN-1 in 20 mM phosphate buffer (pH 7.4) containing 50% methanol and 50 µM DETAPAC under aerobic conditions at 37 °C for 1 hour. Authentic 4-NO-edaravone (B) and 4-NO2-edaravone (C) eluted at 7.9 and 13.8 minutes, respectively, under identical HPLC conditions. Chromatographic peaks were monitored at m/z = 202.0 and 218.0, respectively.
Figure 3.
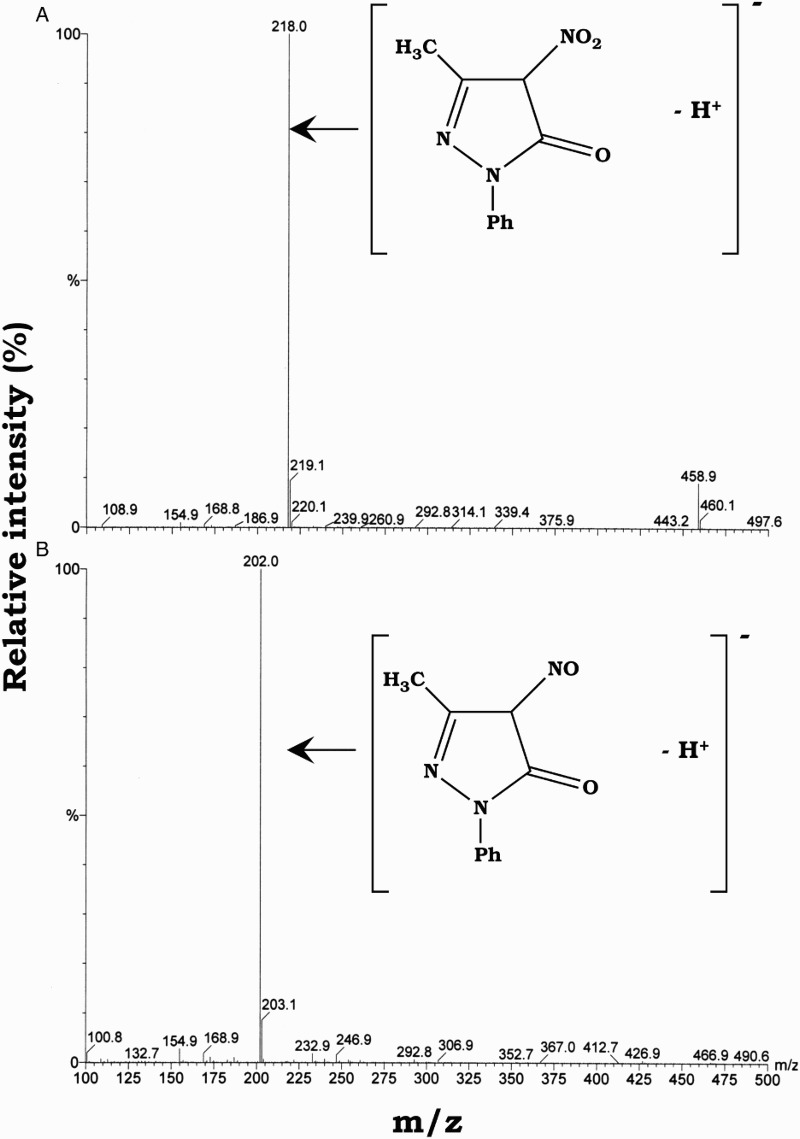
MS spectra of the products eluted at 7.9 (A) and 13.8 minutes (B), identified as 4-NO-edaravone and 4-NO2-edaravone, respectively.
Electrophilic addition of peroxynitrite at the C4 position of edaravone gives 4-NO-edaravone and 4-NO2-edaravone as shown in equations (1) and (2). We deduce that equation (1) is the major pathway of this reaction since 4-NO-edaravone is the predominant product.
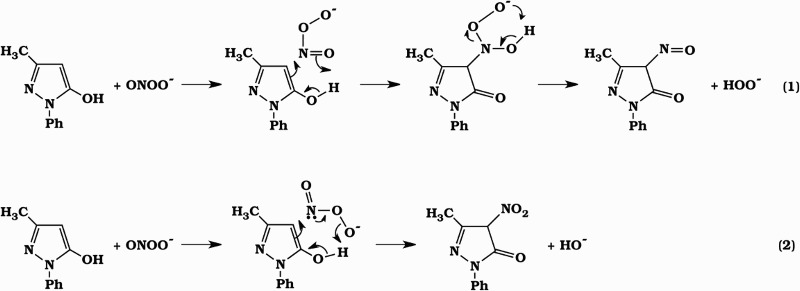
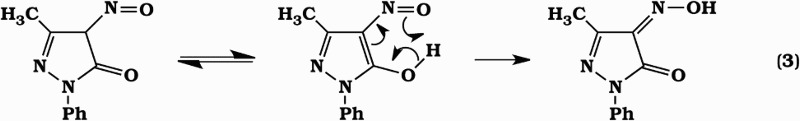
Figure 4 shows the time courses of edaravone, 4-NO-edaravone, 4-NO2-edaravone, 4-oxoedaravone, and OPB concentrations when 1.0 mM edaravone was incubated with 1.0 mM SIN-1 in 20 mM phosphate buffer (pH 7.4) containing 50% methanol and 50 µM DETAPAC under aerobic conditions at 37 °C. 4-NO-edaravone was the major product of this reaction. However, its yield accounted for only 30–40% of the amount of edaravone consumed. This discrepancy may be ascribed to poor quantification caused by tailing of 4-NO-edaravone chromatographic peak as shown in Fig. 2A and B. This peak tailing may be caused by isomerization equilibrium of the NO adduct to its oxime form (equation (3)), which would be expected to interact strongly with the HPLC analytical dimethylamino column.
Figure 4.
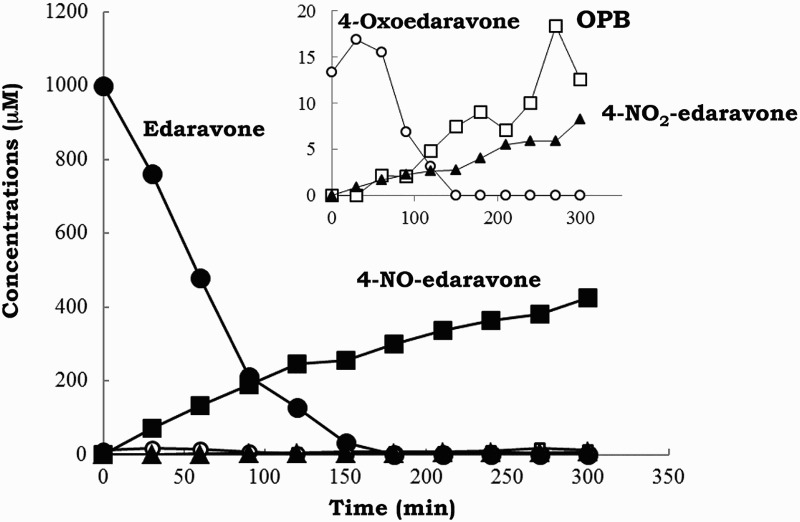
Time course of changes in the concentrations of edaravone (•) and 4-NO-edaravone (▪) when 1.0 mM edaravone was treated with 1.0 mM SIN-1 in 20 mM phosphate buffer (pH 7.4) containing 50% methanol and 50 µM DETAPAC under aerobic conditions at 37 °C. The inset figure shows changes in 4-NO2-edaravone (▴), 4-oxoedaravone (○), and OPB (□).
4-Oxoedaravone, the free radical oxidation product of edaravone, was present at time 0 prior to the reaction onset of edaravone with peroxynitrite and is attributed to be an impurity of commercially available edaravone. All of the 4-oxoedaravone slowly hydrolyzed to OPB (Fig. 4). These results indicate that formation of hydroxyl radical (•OH) and nitrogen dioxide (•NO2) by dissociation of protonated peroxynitrite (ONOOH)18,25,26 was not involved in the reaction of edaravone with peroxynitrite.
In studies of the pathogenesis of many disease states, it is often necessary to distinguish the roles of free radical-induced oxidation of lipids and peroxynitrite formation in vivo. Clinical dosing of edaravone followed by detection of 4-oxoedaravone (or OPB) and 4-NO-edaravone, respectively, might be good markers for this purpose.
Reactivity of edaravone with peroxynitrite
To examine the reactivity of edaravone with peroxynitrite, we compared the rate constant of edaravone with that of uric acid, 480 M−1 s−1.21 Fig. 5 shows the decay of edaravone and uric acid, when equimolar concentrations of the two substrates were treated with peroxynitrite. The decrease of edaravone was much faster than that of uric acid, and the ratio of reactivity of edaravone to that of uric acid was calculated to be 30.6 ± 5.2 (n = 5). From this we calculated the rate constant for the reaction of edaravone with peroxynitrite to be approximately 1.5 × 104 M−1 s−1. The physiological concentration of uric acid in healthy human plasma is about 300 µM and an achievable concentration of edaravone is 50 µM.3 In such condition, edaravone is expected to scavenge peroxynitrite about 5 (=30 × 50/300) times faster than does uric acid.
Figure 5.
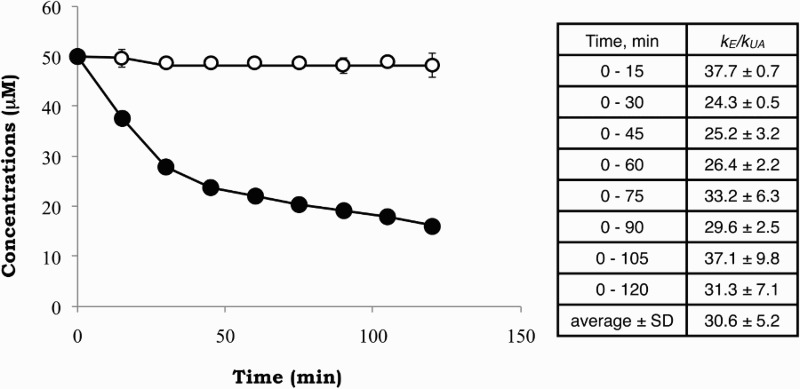
Time course in the decay of edaravone (•) and uric acid (○) when 50 µM of each compound was admixed with 50 µM SIN-1 in 20 mM phosphate buffer (pH 7.4) containing 50% methanol and 50 µM DETAPAC under aerobic conditions at 37 °C. The inset table gives kE/kUA calculated from five independent measurements.
Potential targets of edaravone treatment
Reduced plasma (serum) levels of uric acid, one of the most important endogenous peroxynitrite scavenger, have been reported in patients with multiple sclerosis, Parkinson's disease, Alzheimer's disease, and optic neuritis,27 as well as amyotrophic lateral sclerosis (ALS).28 Edaravone has been reported to alleviate progression of ALS29 and this is now under investigation at a clinical scale. We believe scavenging of peroxynitrite by edaravone contributes significantly to edaravone's efficacy, since 3-nitrotyrosine levels in the cerebrospinal fluid of patients with ALS are reported to be lower by this treatment.29 Edaravone treatment of ALS patients is found to increase their plasma uric acid level.30 Therefore, edaravone may be therapeutically useful in various diseases that present with increased 3-nitrotyrosine levels in plasma and tissues18 and/or low plasma uric acid.
In conclusion, we found the free radical scavenger drug, edaravone, reacts strongly with peroxynitrite to produce 4-NO-edaravone as its major product. Furthermore, this electrophilic addition of peroxynitrite does not accompany the formation of damaging free radicals such as hydroxyl radical and nitrogen dioxide. Subsequent to edaravone administration, 4-NO-edaravone can be used accordingly as a footprint of peroxynitrite formation in vivo. The reactivity of edaravone towards peroxynitrite is approximately 30 times greater than that of uric acid, suggesting the usefulness of edaravone treatment for patients with high 3-nitrotyrosine and/or low plasma uric acid levels.
Acknowledgments
A gift of 4-oxoedaravone, OPB, 4-NO-edaravone, and 4-NO2-edaravone from Mitsubishi Tanabe Pharma Corporation is gratefully acknowledged. We thank Dr Walter C. Dunlap and Dr Kazutoshi Watanabe for their valuable contribution to the presentation of this manuscript.
Disclaimer statements
Contributors All authors contributed equally.
Funding None.
Conflict of interest We have not received any financial support or other benefits from commercial sources for the work reported here. None of the authors has financial interests that could create a potential conflict of interest or the appearance of a conflict of interest with regard to this work.
Ethics approval Not applicable.
References
- 1.Schmidley JW. Free radicals in central nervous system ischemia. Stroke 1990;21:1086–90. doi: 10.1161/01.STR.21.7.1086 [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem 1992;59:1609–23. doi: 10.1111/j.1471-4159.1992.tb10990.x [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto Y, Kuwahara T, Watanabe K, Watanabe K. Antioxidant activity of 3-methyl-1-phenyl-2-pyrazolin-5-one (MCI-186). Redox Rep 1996;2:333–8. [DOI] [PubMed] [Google Scholar]
- 4.Edaravone Acute Infarction Study Group Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction: randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis 2003;15:222–9. doi: 10.1159/000069318 [DOI] [PubMed] [Google Scholar]
- 5.Watanabe K, Watanabe K, Kuwahara T, Yamamoto Y. Free radical-induced oxidation products of 3-methyl-1-phenyl-2-pyrazolin-5-one (MCI-186). J Jpn Oil Chem Soc 1997;46:797–801. doi: 10.5650/jos1996.46.797 [DOI] [Google Scholar]
- 6.Abe K, Yuki S, Kogure K. Strong attenuation of ischemic and postischemic brain edema in rats by a novel free radical scavenger. Stroke 1988;19:480–5. doi: 10.1161/01.STR.19.4.480 [DOI] [PubMed] [Google Scholar]
- 7.Shichinohe H, Kuroda S, Yasuda H, Ishikawa T, Iwai M, Horiuchi M, et al. . Neuroprotective effects of the free radical scavenger edaravone (MCI-186) in mice permanent focal brain ischemia. Brain Res 2004;1029:200–6. doi: 10.1016/j.brainres.2004.09.055 [DOI] [PubMed] [Google Scholar]
- 8.Zhang N, Komine-Kobayashi M, Tanaka R, Liu M, Mizuno Y, Urabe T. Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke 2005;36:2220–5. doi: 10.1161/01.STR.0000182241.07096.06 [DOI] [PubMed] [Google Scholar]
- 9.Kawai H, Nakai H, Suga M, Yuki S, Watanabe T, Saito K. Effects of a novel free radical scavenger, MCl-186, on ischemic brain damage in the rat distal middle cerebral artery occlusion model. J Pharmacol Exp Ther 1997;281:921–7. [PubMed] [Google Scholar]
- 10.Yamamoto Y, Yanagisawa M, Tak NW, Watanabe K, Takahashi C, Fujisawa A, et al. . Repeated edaravone treatment reduces oxidative cell damage in rat brain induced by middle cerebral artery occlusion. Redox Rep 2009;14:251–8. doi: 10.1179/135100009X12525712409779 [DOI] [PubMed] [Google Scholar]
- 11.Love S. Oxidative stress in brain ischemia. Brain Pathol 1999;9:119–31. doi: 10.1111/j.1750-3639.1999.tb00214.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szabó G, Loganathan S, Merkely B, Groves JT, Karck M, Szabó C, et al. . Catalytic peroxynitrite decomposition improves reperfusion injury after heart transplantation. J Thorac Cardiovasc Surg 2012;143:1443–9. doi: 10.1016/j.jtcvs.2012.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suofu Y, Clark J, Broderick J, Wagner KR, Tomsick T, Sa Y, et al. . Peroxynitrite decomposition catalyst prevents matrix metalloproteinase activation and neurovascular injury after prolonged cerebral ischemia in rats. J Neurochem 2010;115:1266–76. doi: 10.1111/j.1471-4159.2010.07026.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Chen CL, Kang PT, Garg V, Hu K, Green-Church KB, et al. . Peroxynitrite-mediated oxidative modifications of complex II: relevance in myocardial infarction. Biochemistry 2010;49:2529–39. doi: 10.1021/bi9018237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker LM, Walker PD, Imam SZ, Ali SF, Mayeux PR. Evidence for peroxynitrite formation in renal ischemia–reperfusion injury: studies with the inducible nitric oxide synthase inhibitor l-N(6)-(1-iminoethyl)lysine. J Pharmacol Exp Ther 2000;295:417–22. [PubMed] [Google Scholar]
- 16.Guven A, Uysal B, Akgul O, Cermik H, Gundogdu G, Surer I, et al. . Scavenging of peroxynitrite reduces renal ischemia/reperfusion injury. Ren Fail 2008;30:747–54. doi: 10.1080/08860220802213039 [DOI] [PubMed] [Google Scholar]
- 17.Nauser T, Koppenol WH. The rate constant of the reaction of superoxide with nitrogen monoxide: approaching the diffusion limit. J Phys Chem A 2002;106:4084–6. doi: 10.1021/jp025518z [DOI] [Google Scholar]
- 18.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007;87:315–424. doi: 10.1152/physrev.00029.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banno M, Mizuno T, Kato H, Zhang G, Kawanokuchi J, Wang J, et al. . The radical scavenger edaravone prevents oxidative neurotoxicity induced by peroxynitrite and activated microglia. Neuropharmacology 2005;48:283–90. doi: 10.1016/j.neuropharm.2004.10.002 [DOI] [PubMed] [Google Scholar]
- 20.Dohare P, Hyzinski-García MC, Vipani A, Bowens NH, Nalwalk JW, Feustel PJ, et al. . The neuroprotective properties of the superoxide dismutase mimetic tempol correlate with its ability to reduce pathological glutamate release in a rodent model of stroke. Free Radic Biol Med 2014;77:168–82. doi: 10.1016/j.freeradbiomed.2014.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos CX, Anjos EI, Augusto O. Uric acid oxidation by peroxynitrite: multiple reactions, free radical formation, and amplification of lipid oxidation. Arch Biochem Biophys 1999;37:285–94. doi: 10.1006/abbi.1999.1491 [DOI] [PubMed] [Google Scholar]
- 22.Tsukada K, Hasegawa T, Tsutsumi S, Katoh H, Kuwano H, Miyazaki T, et al. . Effect of uric acid on liver injury during hemorrhagic shock. Surgery 2000;127:439–46. doi: 10.1067/msy.2000.104486 [DOI] [PubMed] [Google Scholar]
- 23.Scott GS, Cuzzocrea S, Genovese T, Koprowski H, Hooper DC. Uric acid protects against secondary damage after spinal cord injury. Proc Natl Acad Sci U S A 2005;102:3483–8. doi: 10.1073/pnas.0500307102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato Y, Kawakishi S, Aoki T, Itakura K, Osawa T. Oxidative modification of tryptophan residues exposed to peroxynitrite. Biochem Biophys Res Commun 1997;234:82–4. doi: 10.1006/bbrc.1997.6587 [DOI] [PubMed] [Google Scholar]
- 25.Radi R. Peroxynitrite, a stealthy biological oxidant. J Biol Chem 2013;288:26464–72. doi: 10.1074/jbc.R113.472936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carballal S, Bartesaghi S, Radi R. Kinetic and mechanistic considerations to assess the biological fate of peroxynitrite. Biochim Biophys Acta 2014;1840:768–80. doi: 10.1016/j.bbagen.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kutzing MK, Firestein BL. Altered uric acid levels and disease states. J Pharmacol Exp Ther 2008;324:1–7. doi: 10.1124/jpet.107.129031 [DOI] [PubMed] [Google Scholar]
- 28.Keizman D, Ish-Shalom M, Berliner S, Maimon N, Vered Y, Artamonov I, et al. . Low uric acid levels in serum of patients with ALS: further evidence for oxidative stress? J Neurol Sci 2009;285:95–9. doi: 10.1016/j.jns.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 29.Yoshino H, Kimura A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (Phase II study). Amyotroph Lateral Scler 2006;7:241–5. doi: 10.1080/17482960600881870 [DOI] [PubMed] [Google Scholar]
- 30.Nagase M, Yamamoto Y, Miyazaki Y, Yoshino H. Increased oxidative stress in patients with amyotrophic lateral sclerosis and the effect of edaravone administration. Redox Rep, 2015;[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


