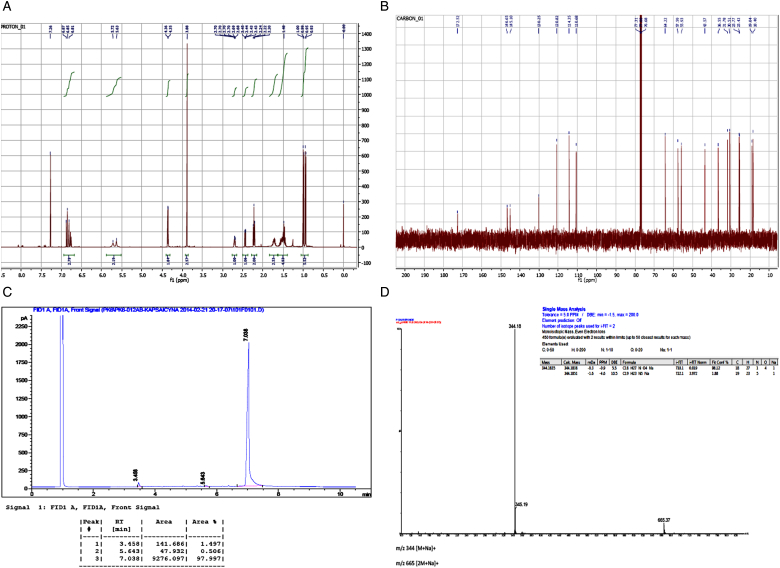
Figure 2.
Capsaicin epoxide characteristics. The quality (purity) and molecular mass of the synthesized capsaicin epoxide (N-(4-hydroxy-3-methoxybenzyl)-5-[3-(propan-2-yl)oxiran-2-yl]pentanamide, compound 2) were evaluated by NMR spectroscopy (A and B), gas chromatography (C), and ESI-TOF mass spectrometry (D). (A) The 400-MHz 1H NMR spectrum of compound 2. (B) The 100-MHz 13C NMR spectrum of compound 2. 1H NMR: (400 MHz) δ (ppm): 0.94 and 1.00 (2d, J = 6 Hz, 6H, –CH(CH3)2), 1.40–1.60 (m, 4H, –CH2–), 1.66–1.79 (m, 3H, –CH2– and –CH(CH3)2, 2.22 (t, J = 8 Hz, 2H, –CH2C=O), 2.44 (dd, J = 4 Hz, 1H, –CH–O–), 2.68–2.72 (m, 1H, –CH–O–), 3.88 (s, 3H, –OCH3), 3.36 (d, J = 4 Hz, 2H, –CH2–NH–), 5.63 (br s, 1H, –OH or –NH–), 5.72 (br s, 1H, –OH or –NH–), 6.75–6.87 (m, 3H, aromatic H).13C NMR: (100 MHz) δ (ppm): 18.4, 19.0 (2C, –CH(CH3)2), 25.4, 25.8, 30.5, 31.8 (4C, –CH2–), 36.6 (1C, –CH(CH3)2, 43.6 (1C, –CH2–NH–), 55.9, 57.6 (2C, epoxide C), 64.2 (1C, –OCH3), 110.7, 114.4, 120.8, 130.3, 145.1, 146.7 (6C, aromatic C), 172.5 (1C, =C=O). (C) The gas chromatographic analysis of compound 2: Rt = 7.038 minutes (98%). (D) ESI (HR MS) data spectrum of compound 2 (calculated for C18H27NO4Na (MNa+): 344.1838; found: 344.1835).