Figure 1.
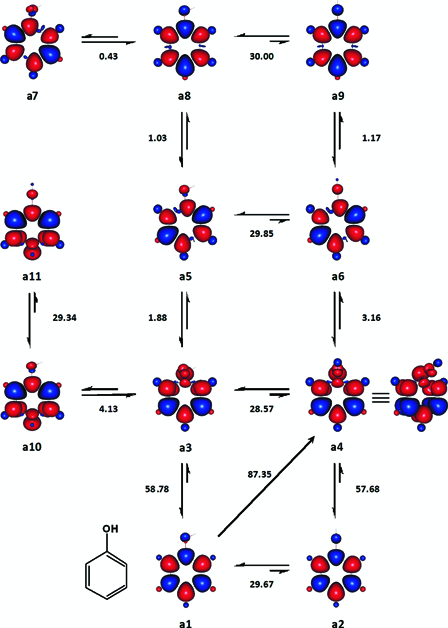
Enthalpy changes between selected states of phenol, which were considered for the estimation of its BDE (kcal mol−1). Enthalpy changes (ΔH°(298 K)) are negative in the direction of the conformer the formation of which is favoured and conversely positive in the opposite direction. Red and blue are spin up and spin down regions, respectively.
