Figure 6.
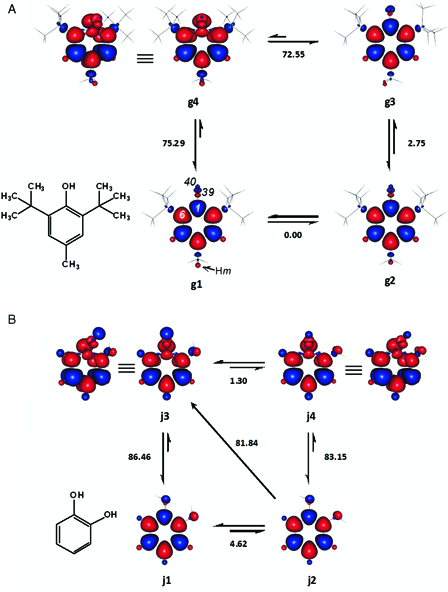
Enthalpy changes (kcal mol−1) between the important conformers of BHT (2,6-di-tert-butyl-4-methylphenol) (A) and catechol (B). In BHT, one of the para methyl hydrogen atoms (Hm) is perpendicular to the average plane of the aromatic ring. Hm is above or below the ring in g1 and g2, respectively. Red and blue are spin up and spin down regions, respectively.
