Figure 2.
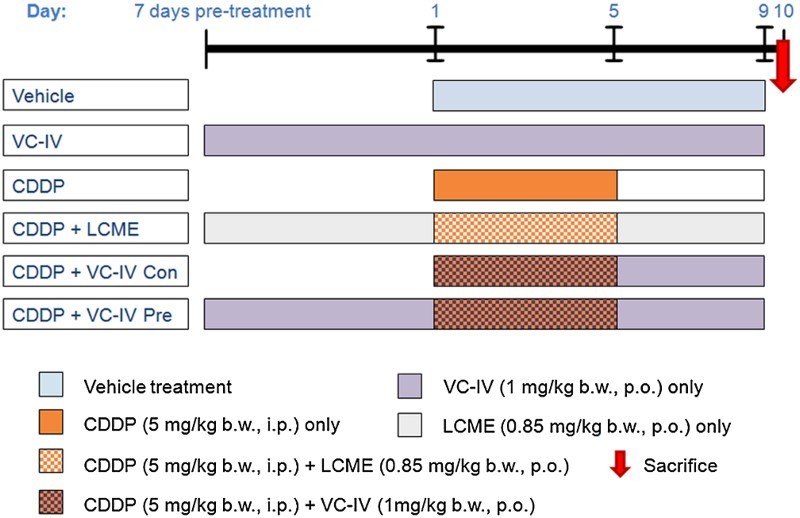
Experimental treatment schedule to study the protective effect of VC-IV against CDDP-induced nephrotoxicity. In the first group the vehicle (5.5% propylene glycol aqueous suspension) was administered for 9 consecutive days. In the VC-IV-treated group, VC-IV was administered at the dose of 1 mg/kg b.w., p.o. throughout the period (16 days). In the CDDP-treated group, CDDP (5 mg/kg b.w., i.p.) was administered for consecutive 5 days (day 1 to day 5). In the fourth group, the ligand LCME (0.85 mg/kg b.w., p.o.) was administered along with CDDP in order to study the effect of the ligand only. In the fifth and sixth group, the organovanadium complex (1 mg/kg b.w., p.o.) was administered along with CDDP in concomitant-treatment and 7 days pre-treatment schedule, respectively. VC-IV: oxovanadium (IV)-L-cysteine methyl ester complex; CDDP: cisplatin; LCME: L-cysteine methyl ester hydrochloride.
