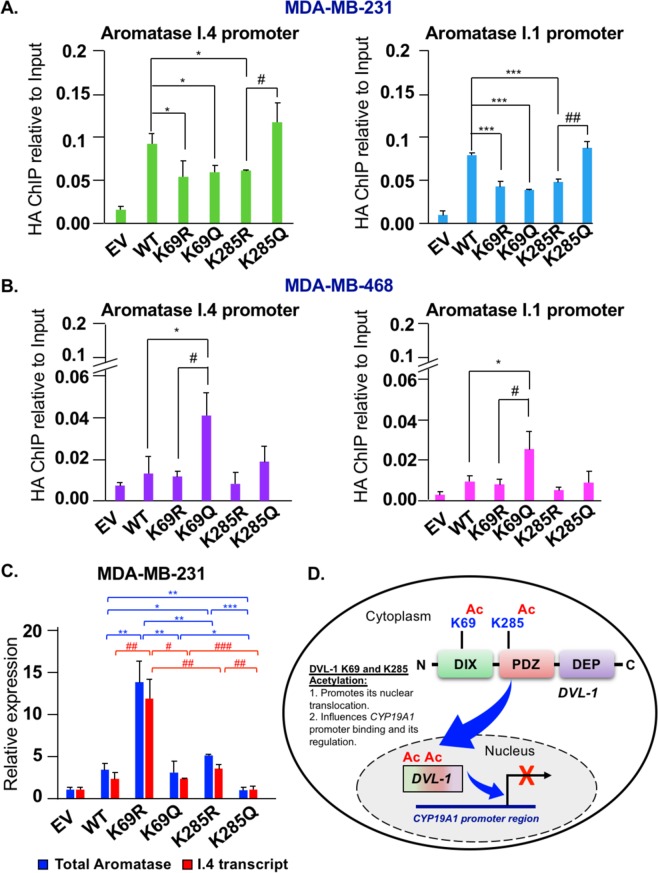
Figure 8.
DVL-1 acetylation on K69 and K285 lysine residues influences its binding and regulation of CYP19A1 (aromatase) tissue-specific promoters. Occupancy of HA-tagged DVL-1 at tissue-specific promoters of CYP19A1 gene (I.4, and I.1) were analysed by real-time PCR. ChIP experiments for HA-tag were performed in (A) MDA-MB-231 and (B) MDA-MB-468 cells stably expressing EV (empty vector; negative control), HA-tagged wild-type DVL-1 (WT), HA-tagged deacetylation mutants (K69R and K285R), and HA-tagged acetylation mutants (K69Q and K285Q). The sign “*” represents significant change in HA-tagged DVL-1 binding between WT and mutants, while “#” represents significant change between R and Q mutants. (C) RNA was isolated from MDA-MB-231 cells stably expressing EV, WT, K69R/Q, and K285R/Q and cDNA was synthesized. Quantitative PCR was then performed using primers specific for adipose I.4 aromatase transcript (red), and the total aromatase (blue) mRNA with primers in the coding region common to all transcripts. (D) Schematic representation of the functional significance of acetylation on DVL-1 nuclear localization and its impact on binding and regulation of target genes such as CYP19A1 promoter region. All results are expressed as mean ± SEM and considered significant at */#p < 0.05, **/##p < 0.01 and ***/###p < 0.001.