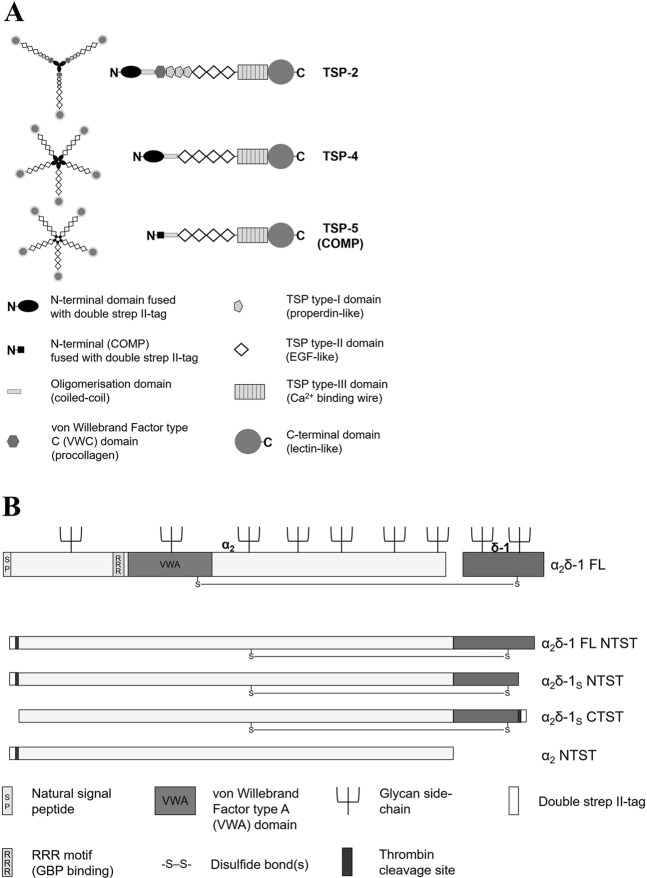
Figure 1.
Schematic presentation of the structures of the recombinant proteins generated in this study. (A) Domain structure and oligomerisation state of the generated recombinant full-length TSP-2 (trimer), TSP-4 and COMP (pentamers). Schematic representation adapted by permission from Springer Nature, Cell Mol Life Sci, Structures of thrombospondins, Carlson, C. B., Lawler, J. & Mosher, D. F., Copyright (2008)39. All recombinant TSPs have been expressed with an N-terminal double strep II-tag and contain glycan side-chains which are not shown for reasons of clarity. (B) Structure of α2δ-1 FL protein (adapted from Cell 139, Eroglu, Ç. et al., Gabapentin receptor αδ2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis, 380–392, Copyright (2009), with permission from Elsevier7) and simplified depiction of the derived non-proteolytically processed α2δ-1 mutants generated in this study. The RRR motif, the von Willebrand Factor type A domain, and the glycan side-chains are not shown in the α2δ-1 mutants for reasons of clarity.