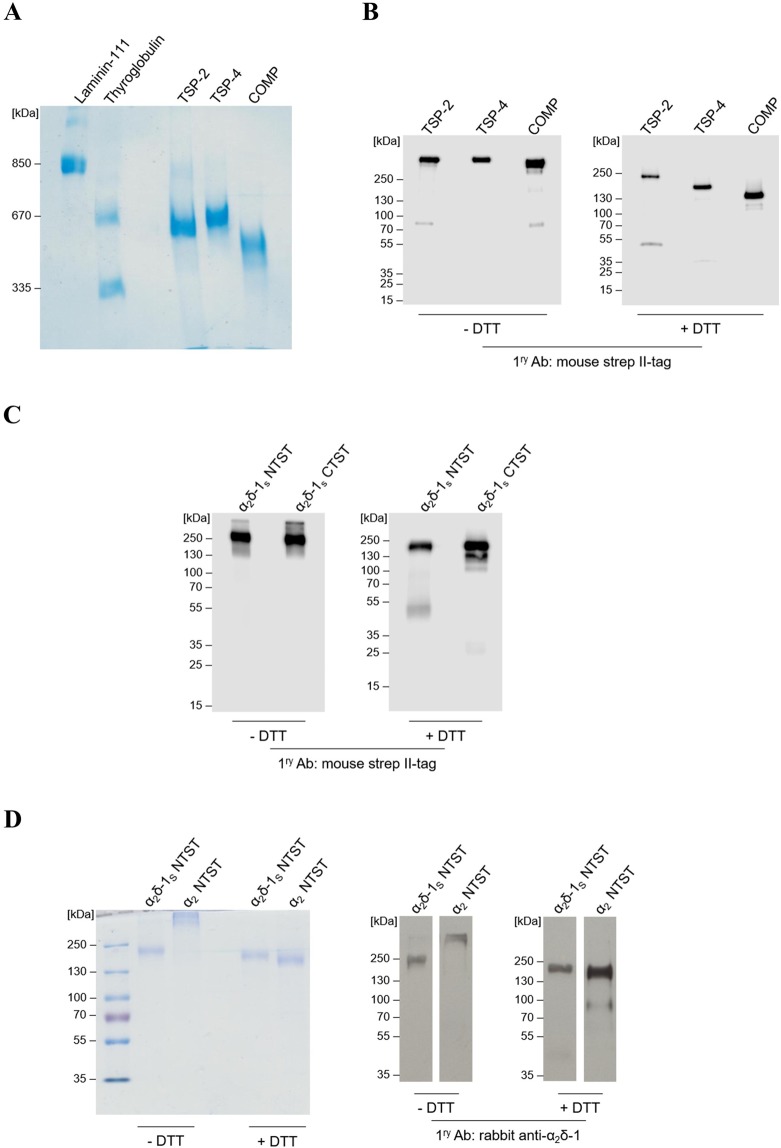
Figure 2.
The generated recombinant TSPs and α2δ-1S variants show high degree of purity and integrity in Coomassie staining and western blot analyses. (A,D left) Representative Coomassie-stained gels and (B,C and D right) immunoblots of three full-length TSP proteins, all carrying an N-terminal double strep II-tag: TSP-2, TSP-4, and COMP (A,B); α2δ-1S variants carrying either an N-terminal (α2δ-1S NTST) or a C-terminal (α2δ-1S CTST) double strep II-tag (C); α2δ-1S NTST and α2 peptide chain carrying an N-terminal double strep II-tag, α2 NTST (D). Proteins were separated under non-reducing (−DTT) or reducing conditions (+DTT) on 4–15% (B), 10% (C), and 7% (D) polyacrylamide gels, respectively, while in (A) proteins were separated on 0.5% agarose (w/v)/3% polyacrylamide (w/v) composite gels without prior DTT treatment. Proteins were either stained with colloidal Coomassie stain (A,D left) or detected with the following primary antibodies after blotting: mouse anti-strep II-tag (B,C) or rabbit anti-α2δ-1 (D right). Secondary antibodies included the polyclonal rabbit anti-mouse IgG (B,C) and swine anti-rabbit IgG (D right), both conjugated with horseradish peroxidase (see Supplementary Table S2 for further information). In all gels the molecular weight standard (in kDa) indicated on the left was PageRuler Plus Prestained Protein Ladder (Thermo Fisher Scientific) except for (A) in which both thyroglobulin (Sigma) and recombinant laminin-111 (kind gift from Prof. Dr. Monique Aumailley, Institute for Biochemistry II, Centre for Biochemistry, Medical Faculty, University of Cologne) were used.