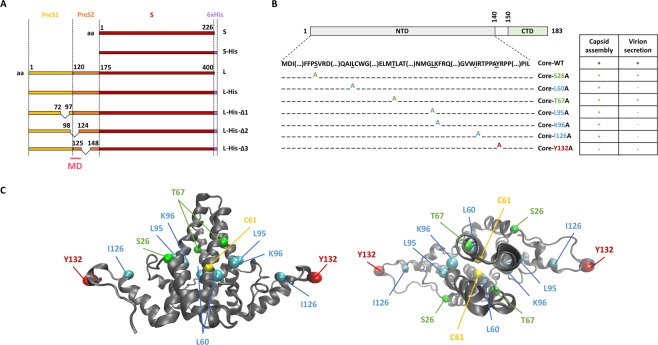
Figure 1.
Diagram of the S, L and core proteins and their mutated derivatives. (A) The WT S envelope protein consists of the S domain (red) and the WT L envelope protein consists of this domain together with two other domains at its N-terminus: the preS1 (yellow) and the preS2 (orange) domains. The matrix domain (MD) is indicated (pink). Deletions are indicated by a bridge linking the flanking residues. Some constructs contain a His-tag at their C-terminus (purple box). (B) The WT core protein consists of the NTD domain (light gray), a linker (white) and the CTD domain (green). Seven mutant core proteins were generated by alanine substitution. The ability of mutated core proteins to assemble and to produce virions, according to the findings of Ponsel et al.18, is summarized in the table on the right. The names of the constructs are shown in color, according to their capsid assembly and virion secretion properties: C+V+ residues in green, C+V− residues in blue, C-V- residues in red. (C) Orthogonal views of the 3D structure of the core NTD dimer linked by C61 residues (in yellow). This structure was obtained by crystallography (#1QGT)11. The backbone is represented as a gray ribbon. Mutated residues are shown in the CPK model, with colors as in the table in (B).