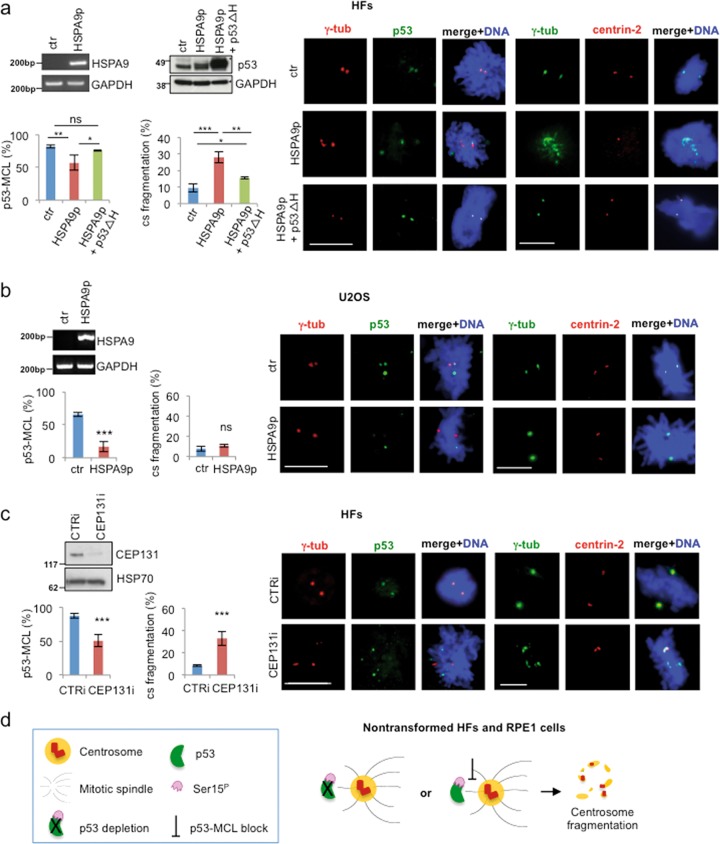
Fig. 3. Centrosome fragmentation is linked to impaired p53-MCL.
a HFs were transfected with the empty vector (ctr), the vector expressing the HSPA9253–282 peptide (HSPA9p) and analyzed by semiquantitative RT-PCR since no Abs are available for the detection of the small peptide. In addition, HFs were co-transfected with the HSPA9p-expressing vector and a second vector encoding for human p53-Δ(325–355), missing the region required to bind HSPA9 protein (p53ΔH) and analyzed by WB. p53-MCL was analyzed by double IF for γ-tubulin/p53 and centrosome structure by double IF for γ-tubulin/centrin-2, as described in Fig. 2. Representative IF images and histograms show that HSPA9p expression impairs p53-MCL and induces centrosome fragmentation. At variance from p53i HFs, in these conditions, p53 is still present but does not colocalize with γ-tubulin. When p53-MCL was restored by p53ΔH expression, centrosome fragmentation was inhibited, as shown by representative IF images and relative histograms. These experiments were repeated three times and at least 200 mitoses per sample were analyzed. b U2OS cells were transfected with the empty vector (ctr) and the vector expressing the HSPA9p and analyzed as described in (a). In these cells, p53-MCL was even more significantly reduced than in HFs, but no sign of centrosome fragmentation was detected. These experiments were repeated three times and 300 mitoses per sample were analyzed. c HFs were transfected with CTRi and CEP131i siRNAs, analyzed for CEP131 expression by WB and for p53-MCL and centrosome structure and number as above. Representative IF images and histograms show that CEP131-depletion inhibits p53-MCL and induces centrosome fragmentation. These experiments were repeated four times and at least 200 mitoses per sample analyzed. ns = not significant; *P < 0.05; **P < 0.01; ***P < 0.001. Scale bars, 10 µm. d Schematic representation of the results obtained in nontransformed human cells by blocking p53-MCL (i.e., p53 depletion or impairment of p53 centrosomal localization)